Method correlates solubilities of crude hydrocarbons in water
A new correlation calculates the water solubility for a wide variety of hydrocarbons found in a typical crude oil-alkanes, olefins, diolefins, acetylenes, cyclopentanes, cyclohexanes, benzenes, mercaptans, thiophenes, and sulfides.
The correlation provides reliable solubility values down to extremely low concentrations, in the ppm range. It agrees favorably with experimental data.
Results from the new correlation are useful for process engineering for wastewater minimization.
Importance of water solubility
Knowing the solubility of crude oil hydrocarbons in water is important for health, safety, and environmental considerations, an importance that will only increase with time.
For health involving human exposure to substances in air, the threshold limit value (TLV) for pentane in air is 600 ppm (vol), according to the US Occupational Safety and Health Administration.1 A concentration of only 0.0000001 mole fraction of pentane in water will provide about 7,000 ppm (vol) of pentane in air at the air-water interface, which greatly exceeds the 600 ppm (vol) TLV.
To ensure safe operations, a lower explosion limit (LEL) for pentane in air is reportedly 1.4%.2 A concentration of only 0.000001 mole fraction of pentane in water provides about 7% of pentane in air at the air-water interface.
To illustrate environmental effects, consider a spill of pentane in contact with water. The water will become saturated; at saturation, the solubility of pentane in water is about 0.0000385 weight fraction, or 0.00000916 mole fraction, according to Yalkowski.3
This concentration at saturation leads to a pentane level of 64.4% in air at the air-water interface, which is considerably higher than the TLV and LEL.
Correlation for solubility
In an earlier work by Yaws and coworkers, water solubility for various chemical types was correlation as a function of the boiling point temperature of the compound.2
For the new correlation, we determined that the boiling point method was also applicable for correlating water solubility of crude oil hydrocarbons. Equation 1 (see accompanying equation box) shows the new correlation.
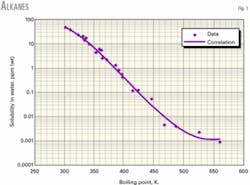
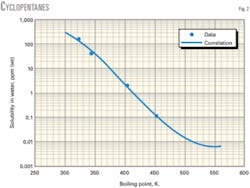
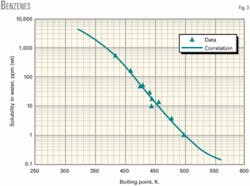
The range of boiling point temperatures for the hydrocarbons studied was 298-560 K.
Table 1 shows the regression coefficients for a wide variety of hydrocarbons: alkanes, olefins, diolefins, acetylenes, cyclopentanes, cyclohexanes, benzenes, mercaptans, thiophenes, and sulfides. The table also shows the boiling point range (minimum, maximum) for which the correlation is applicable. The correlation should not be used for compounds with boiling points outside the boiling point range.
We determined the coefficients using regressions of available data. In preparing the correlation, we conducted a literature search to identify data source publications.2-12 The excellent compilations by Howard and Meylan;7 Mackay, Shiu, and Ma;8 Verschueren;10 Yalkowski;3 and Yaws2 were used extensively for water solubility.
The boiling point temperatures are from compilations from Yaws.2 11 12
The publications were screened and copies of the appropriate data were made. These data were then keyed in to the computer to provide a database for which experimental data are available. The database also served as a basis to check the accuracy of the correlation.
Figs. 1-3 show water solubility vs. boiling point temperature for representative hydrocarbon families. The graphs indicate favorable agreement of correlation values and experimental data.
Example
A chemical spill of toluene occurs into a body of water at ambient conditions. Estimate the concentration in the water at saturation.
The correlation for benzenes (single substitution) can be used to determine the solubility in water. Substitution of the coefficients and boiling point temperature of toluene into Equation 1 yields:
log10(S) = -24.0080 + 0.221196 × (383.78) + 5.55632E-4 × (383.73)2 + 4.1883E-7 × (383.73)3
S = 524.68 ppm (wt)
The calculated value and experimental data compare favorably: 524.68 vs. 542.4. Deviation is (542.4-524.68)/542.4, or 3.3% error.
References
- OSHA, “Guide to Occupational Exposure Values-1997,” American Conference of Governmental Industrial Hygienists, ACGIH, Cincinnati, 1997.
- Yaws, C.L., “Chemical Properties Handbook,” New York: McGraw-Hill Cos., 1999.
- Yalkowski, S.H., “Aquasol Database,” University of Arizona, Tucson, 1990-2004.
- Mackay, D., Shiu, W. Y., and Ma, K. C., “Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals,” Vol. 4, New York: Lewis Publishers, 1995.
- “Handbook of Environmental Data on Organic Chemicals,” 4th Ed., New York: John Wiley & Sons, 2001.
- Hine, J., and Mukherjee, P.K., Journal of Organic Chemistry, Vol. 40 (1975), p. 292.
- Howard, P.H., and Meylan, W.M., eds., “Handbook of Physical Properties of Organic Chemicals,” Boca Raton, Fla.: CRC Press, 1997.
- Mackay, D., Shiu, W. Y., and Ma, K. C., “Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals,” Vols. 1-5, New York: Lewis Publishers, 1992-97.
- Suzuki, T., Journal of Computer-Aided Molecular Design, Vol. 5 (1991), p. 149.
- Verschueren, K., “Handbook of Environmental Data on Organic Chemicals,” 3rd and 4th Eds., New York: Van Nostrand Reinhold, 1996, 2001.
- Yaws, C.L., “Yaws Handbook of Thermodynamic and Physical Properties of Chemical Compounds,” electronic edition, www.knovel.com, Norwich, NY: Knovel, 2003-07.
- Yaws, C.L., “Yaws Handbook of Physical Properties of Hydrocarbons and Chemicals,” Houston: Gulf Publishing Co., 2005.
The author
Carl L. Yaws ([email protected]) is professor of chemical engineering at Lamar University, Beaumont, Tex. His research interests include technology development, thermodynamic and transport property data, environmental engineering, and process simulation. Yaws holds BS in chemical engineering from Texas A&I University, Kingsville, and an MS and PhD in chemical engineering from the University of Houston. He is a registered professional engineer in Texas.