New equations estimate acid-gas solubility in TEG
New equations, which cover the full range of dehydration-plant operating conditions and wide range of experimental data results, estimate the amount of H2S and CO2 absorbed per volume of triethylene glycol (TEG) circulated vs. the partial pressure of acid-gas components and the absorber temperature.
This article provides comparisons with experimental data.
Dehydration process
Glycol dehydration of natural gas employs TEG or diethylene glycol (DEG) to remove water from the gas stream. At low temperatures and high pressures, water vapor can cause hydrate formation or corrosion when it contacts H2S or CO2, components regularly found in produced-gas streams.1
When sour natural gas or acid gas is dehydrated with TEG, substantial amounts of H2S and CO2 are absorbed in the TEG. Regeneration of the rich TEG solution liberates the acid-gas components. The amount of these compounds absorbed and consequently liberated from the glycol depends on their concentrations in the sour gas being dehydrated and on the contactor’s pressure and temperature.
New equations, proposed in this article, apply to pure TEG or to lean TEG, which is essentially pure. These equations show good agreement with experimental data, which were obtained with pure glycol.2
In an actual dehydration facility, the glycol is regenerated to about 99% purity or higher. The absorbed amount of acid gas depends on the amount of glycol circulated. If the glycol is not regenerated to this purity, then the amount of gas absorbed per unit of lean glycol circulated would be slightly less than in the proposed equations.
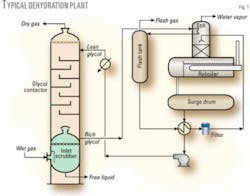
Fig. 1 illustrates a typical dehydration process train. The regenerated glycol is pumped to the top tray of the contactor (absorber). The glycol absorbs water as it flows countercurrently down through the contactor to the gas flow.
Water-rich glycol is removed from the bottom of the contactor, passes through the reflux condenser coil, flashes off most of the soluble gas in the flash tank, and flows through the rich-lean heat exchanger to the regenerator.
In the regenerator, absorbed water is distilled from the glycol at near-atmospheric pressure by application of heat. The regenerated lean glycol leaves the surge drum, is partly cooled in the lean-rich exchanger, and pumped through the glycol cooler before being recirculated to the contactor.1 3
Glycol will absorb some hydrocarbons at the high pressure of the contactor. Glycol has a special affinity for such cyclic hydrocarbons as benzene, toluene, ethyl benzene, and xylene (BTEX) as well as polar gases H2S and CO2 (acid gases).
Heavier paraffinic hydrocarbons are essentially insoluble in TEG. Aromatic hydrocarbons, however, are very soluble in TEG, and significant amounts of aromatic hydrocarbons may be absorbed in TEG at contactor conditions. This may present an environmental or safety hazard when they are discharged from the top of the regenerator.
Upon regeneration of the glycol, all absorbed gases are flashed off. From here they can be routed to fuel, flare, or gas-recovery system.
New equations
Process engineers currently use commercial software to predict the solubility of H2S and CO2 in TEG. These software packages use equations of state and need complete process data to simulate sour-gas dehydration plants and consequently calculate the solubility of acid gases in TEG.
Natural gases containing sour gases exhibit different behavior than do sweet gases. Acid gases are polar components; it is impossible accurately to predict their solubility in TEG by routine commercial software. Moreover, this commercial software is expensive.
Another widely used procedure employs graphics. One such new graph to predict the solubility of Acid gases in TEG has recently been presented.4
Graphs present a convenient visual comparison of the effect of pressure and temperature on the solubility of acid gases in TEG, but these graphs are less accurate in predicting the solubility of acid gases in TEG, especially when they are plotted in log scale.
Moreover, process engineers have difficulty reading a graph accurately, and modern design techniques are usually based on computer calculations. The benefit of equations is that they can easily be programmed and incorporated in the overall design program, thus bypassing the need for charts or graphs.
The proposed new equations have been developed on the basis of experimental data.2 The solubility of H2S and CO2 in TEG is a function of temperature and partial pressure. The partial pressure is the product of the operating pressure times the mole fraction of H2S or CO2 in the gas in the absorber.
The amount of acid gases removed by the glycol depends on the following parameters:
• Concentration of acid gases in the feed gas. The effect of composition increases with pressure, particularly for a gas that contains CO2 or H2S or both.
• Contactor operating pressure and temperature.
• Glycol circulation rate.
The first equation box (p. 55) shows the proposed equations for the solubility of H2S in TEG. The pressure limits of applicability are between 50 kPa (abs) and 2,000 kPa (abs) partial pressure, and temperature is limited to 130º C.
Concerning CO2, as shown in the second equation box (p. 55), two sets of equations are required to cover the pressure operating range. The first set applies to the partial-pressure range between 20 kPa (abs) and 750 kPa (abs), and temperature is limited to 80º C. The second set of equations applies to the partial-pressure range greater than 750 kPa (abs) to 10,000 kPa (abs), and temperature is limited to 130º C.
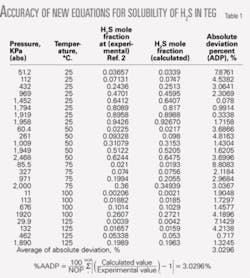
Table 1 shows comparisons between the experimental data for H2S of Reference 2 with results from the equations of the first equation box. As can be seen, the average absolute deviation percent at different temperatures for H2S is 3.0296%.
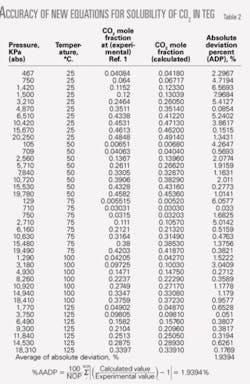
Table 2 shows comparisons between the experimental data for CO2 of Reference 2 with results from the equations of the second equation box. As can be seen, the average absolute deviation percent at different temperatures for CO2 is 1.9394%.
The accuracy of the new proposed equations is acceptable and much better, simpler, and easier to use than routine graphical methods or conventional commercial software.
Results
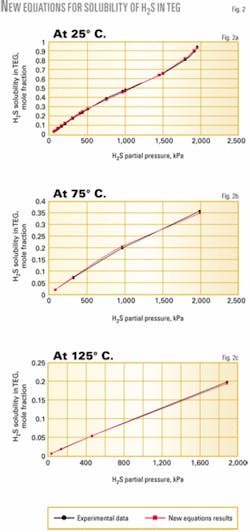
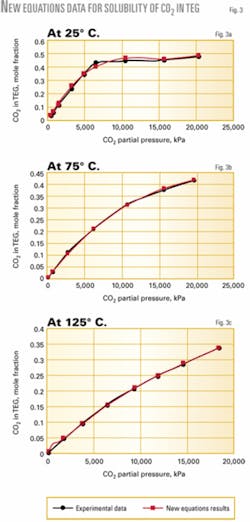
Figs. 2 and 3 show the solubility of H2S and CO2 at different temperatures and partial pressures in TEG. There is a good agreement between the new proposed equations and experimental results.
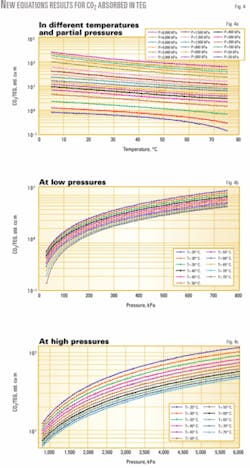
Fig. 4a shows the results of CO2 equations sets, which have been plotted in the log scale. It also shows the trend of solubility of CO2 in TEG at different temperatures and partial pressures. For low temperature and higher pressure, the solubility of CO2 in TEG increases considerably.
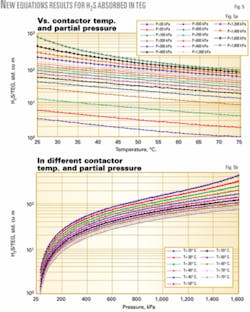
Fig. 5a shows the results of the H2S equations set, which have been plotted in the log scale. It also shows the trend of solubility of H2S in TEG at different temperatures and partial pressures. These new proposed equations are very accurate in predicting the solubility of acid gases in TEG.
Figs. 5b and 4b and c show the variation of partial pressure and temperature on the absorbed gas in TEG in another viewpoint.
Since dehydrators usually operate at temperatures of less than 60° C., there was no practical need to include temperatures higher than 75° C. in graphs of this work.
Acknowledgment
The authors extend special thanks to Edward Wichert for his valuable comments on this work. ✦
References
1. Bahadori, A., “Design of glycol unit for maximum efficiency in Gachsaran Oil Field,” SPE No. 81121, Society of Petroleum Engineers, 12th International Latin American and Caribbean Petroleum Engineering Conference, Port-of-Spain, Trinidad, Apr. 27-30, 2003.
2. Jou, F.-Y., Deshmukh, R.D., Otto, F.D., and Mather, A.E., “Vapor-Liquid Equilibria for Acid gases and Lower Alkanes in Triethylene Glycol,” Fluid Phase Equilibria, Vol. 36 (1987), pp. 121-140.
3. Engineering Data Book, Vol. 2; Tulsa: Gas Processors Suppliers Association, 2004.
4. Wichert, E., and Wichert, G.C., “New Charts estimate acid gas solubility in TEG,” Hydrocarbon Processing, January 2004, pp. 47-48.
The authors
Alireza Bahadori (bahadori.a @nisoc.ir) is a senior process engineer with the National Iranian South Oil Co., Ahwaz City, Iran, and a part time lecturer at the University of Masjed-Soleiman. Previously, he worked for 3 years as a CIS technologist for Aghajari Oil & Gas Co. His expertise includes artificial lift design, production optimization, gas processing, crude oil desalting, and facilities engineering. Bahadori holds a technical diploma (1991) in control instrument services from National Iranian Oil Co. artisan school in Aghajari. He also holds a BSc (1998) in chemical engineering from the Petroleum University of Technology, Iran, and an MSc (2000) in chemical engineering from the University of Shiraz, Iran. He is a member of the Iranian Association of Chemical Engineers.
Khalil Zeidani (kzeidani@ ualberta.ca) is a PhD candidate in petroleum engineering at the University of Alberta, Edmonton. Previously, he worked 4 years as a CIS technologist and 3 years as a process engineer for NIOC. His expertise includes production optimization and surface facilities design. He holds an MEng (2002) in reservoir engineering from the University of Calgary and a BSc (1998) in chemical engineering from the Petroleum University of Technology of Iran. Zeidani also holds a diploma in control instrument services from the National Iranian Oil Co.’s Technical School.