Study outlines US refiners’ options to reduce gasoline benzene levels
A recent study examined the effects of new benzene requirements in US gasoline and refiners’ options for best meeting them. How best to meet the new benzene requirements for all US gasoline depends on the refinery’s configuration, gasoline markets, whether blending ethanol or not, and on hydrogen availability.
For most refiners, benzene reduction will be a compliance issue. A refiner must choose and implement a solution that provides the best value, which will often be the low-cost option.
Solutions will be refinery-specific and determined by the current refinery configuration, the type of reforming unit, the amount of benzene contributed from other blendstocks, amount of ethanol blended, and access to markets that allow trading of intermediate streams. The magnitude of the processing required will depend on the final gasoline benzene limits.
A refiner’s first step in meeting the new benzene requirement is to review the benzene contributions to the pool from each source. Next, the refiner should determine the operating window and roles for the existing assets in light of the new regulations.
These steps involve considering the impact from alternative crudes that may be processed, any future refinery expansion, and any changes in FCC unit operations that affect benzene. Once these preliminary steps are complete, the refiner must evaluate all the possible solutions and determine if one offers better economics and flexibility than another.
Gasoline benzene regulations
Due to new regulations proposed by the US Environmental Protection Agency, all US gasoline will be subject to benzene restrictions at levels far lower than those currently applicable only to reformulated gasoline (RFG). Benzene reduction solutions that worked with RFG may be insufficient to meet the new lower benzene requirements; it will be necessary to examine solutions in the context of the entire refinery while taking into account new regulations on volatile organic compounds (VOCs) and ethanol blending.
New regulations proposed by the EPA under Mobile Sources Air Toxics (MSAT) Phase 2 are expected to reduce benzene in all US gasoline to 0.62 vol % starting Jan. 1, 2011. Previous gasoline regulations focused on reducing benzene to less than 1.0 vol % in RFG, which constitutes about 30-35% of the US gasoline pool.
Benzene in conventional gasoline is currently controlled more indirectly through antibacksliding regulations that limit benzene via exhaust toxics tied, in most cases, to the refinery’s MSAT Phase 1 baseline. Because the new regulations will require more refiners to address benzene, the current solutions may not be adequate to meet the new lower benzene limit.
The main source of benzene in gasoline is reformate and most current benzene reduction solutions focus on preventing benzene formation in the reforming unit by removing benzene precursors from the reforming unit feed. Fewer solutions remove benzene from reformate, either for petrochemical production or for chemical conversion followed by return to the gasoline pool.
When gasoline is limited to 1 vol % in the gasoline pool, it is possible to ignore the level of benzene in FCC naphtha, which is 0.5-1.3 vol % depending on FCC operation, catalyst, and feed. At 0.6-vol % benzene in the gasoline pool, the contribution from FCC naphtha may require refiners to look at more expensive benzene-reduction solutions.
In addition to the expected changes in gasoline benzene there are several other changes that will influence gasoline production and may change the benzene-reduction solutions:
- The renewable fuel standard (RFS) enacted under the 2005 Energy Act requires a staged increase in ethanol blending in the US gasoline pool to 7.5 billion gal in 2012 from 4.0 billion gal in 2006. Ethanol provides octane that can replace reformate octane but ethanol typically adds about 1-1.3 psi of rvp to gasoline, which will affect gasoline blending depending on whether an rvp waiver is allowed for ethanol blending.
The 2005 Energy Act provides the option for gasoline producers to meet the gasoline ethanol requirement by blending physical quantities of ethanol or obtaining ethanol credits from other gasoline producers that blend more ethanol than needed to meet the standard. Although this option should provide greater market flexibility, the EPA has not yet finalized these regulations.
Accompanying the RFS is the elimination of the oxygen requirement in RFG. MTBE liability protection was not included in the 2005 Energy Act and most US gasoline producers plan to discontinue blending MTBE in gasoline.
- Many states, such as Wisconsin, Illinois, and Minnesota, have set ethanol requirements for gasoline sold there; other states are also considering requiring ethanol. Uncertainty about federal and state ethanol regulations, as well as whether all grades of RFG will meet compliance without ethanol, creates significant uncertainty as to how the US gasoline market will deal with ethanol.
- The 2005 Energy Act eliminates the northern-tier VOC requirements for RFG. All RFG must meet the more stringent southern-tier VOC limits, which will affect gasoline production, especially gasoline production with ethanol if there is no rvp waiver.
- The minimum octane of gasoline in PADD 4 may increase to 87 from 85 (R+M)/2.
- Based on the 8-hr ozone and PM 2.5 standards in the National Ambient Air Quality Standards, it is likely that more geographic areas will be out of air quality compliance and required to use RFG or other gasoline reformulation as part of their plans to improve air quality.
The current limit on benzene in regions subject to the Euro III and Euro IV standards is 1 vol %. In addition, all Canadian and Japanese gasoline is subject to the 1.0-vol % limit. Reducing benzene in all US gasoline to 0.6 vol % will make it more difficult for foreign suppliers to provide gasoline for the US market. Imported gasoline has supplied more than 10% of US summer gasoline in the past few years and has helped significantly to stabilize US gasoline supplies during this period of high demand.
Benzene management options
Benzene is managed currently to meet RFG limits and to help manage toxic emissions in the gasoline pool. Reviewing where benzene comes from within the refinery is an important step in determining the best solution for further benzene reduction. Typically, reformate is the largest process stream contributing benzene to the gasoline pool.
Table 1 shows that reformate can make up about 75-80 vol % of gasoline pool benzene. The second largest contributor is FCC naphtha.
The level of benzene produced in the FCC naphtha depends on processing severity, feed, catalyst, and additives used. For example, as FCC operations shift from gasoline to propylene production, the benzene level can increase to 1.3 vol % from 0.5 vol %.
Processing more-difficult crudes results in a more severe FCC unit operation, which can create slightly more benzene in the FCC naphtha. Although reducing FCC unit severity may reduce FCC naphtha benzene to some extent, this comes at a cost in yield or conversion that can adversely affect refinery economics.
Reducing benzene in FCC naphtha via saturation is also uneconomic due to the required investment in fractionation. It often results in an octane loss due to saturation of the many olefins present in this fraction.
Recovering benzene from FCC naphtha is generally also uneconomic because it requires removing olefins and sulfur. The amount of benzene in FCC naphtha, however, will likely affect the amount of benzene reduction required in other gasoline streams.
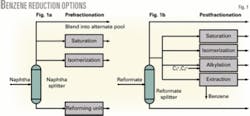
The two basic approaches to reduce benzene from the reforming unit and thereby reduce the benzene content of the gasoline pool are prefractionation and postfractionation.
Prefractionation
In this option, benzene precursors are removed from the reforming unit feed to prevent or reduce benzene formation. Benzene precursors are primarily C6 naphthenes, cyclohexane, methylcyclopentane, and, to a far lesser extent, C6 paraffins.
Benzene precursor removal requires a change in the naphtha cut point and fractionation efficiency. More trays and higher reflux are required to remove most of the C6 cyclics from the reforming unit feed as opposed to typical naphtha splitter designs, which only deisohexanize the reforming unit feed.
Benzene precursor removal typically requires the naphtha splitter to have at least 40 trays. Due to the change in fractionation, the reforming unit feed will contain less C6 cyclics, which results in a reduction in reformate benzene to about 1 vol % from 3-5 vol %.1
With good benzene and benzene precursor removal to the reforming unit, reformate benzene is a function of aromatic dealkylation. At higher pressures, more heavy aromatics are dealkylated to benzene, which may offset the benefits achieved via benzene precursor removal.
Prefractionation results in a higher concentration of benzene in the light naphtha. Depending on which other streams are blended into the gasoline pool, light naphtha may be either blended directly into the gasoline pool or processed for octane enhancement or benzene removal.
Fig. 1a shows the possible naphtha processing options with prefractionation:
- Blending. With a small incremental volume contribution to the gasoline pool and no need for octane, the simplest way to reduce benzene is to fractionate out precursors and blend the light naphtha to the gasoline pool.
- Saturation. When light naphtha contributes too much benzene but there is no need for additional octane, saturation of benzene in the light naphtha may be sufficient.
- Isomerization. When benzene must be reduced and there is a need for nonaromatic octane, refiners should consider light naphtha isomerization.
Postfractionation
This offers the best control of gasoline benzene. In postfractionation, reformate is split into a light and heavy stream in a reformate splitter that typically has 40-50 trays. Benzene is concentrated into the light reformate, and the heavy reformate is blended directly into the gasoline pool.1
Fig. 1b shows the postfractionation options to manage the benzene content in the light reformate. In addition to saturating benzene in the light reformate or isomerizing light reformate, which also saturates benzene, there are two more options-benzene alkylation and extraction:
- Alkylation. Benzene is converted to heavier aromatics via reaction with propylene or ethylene. The advantage of alkylation is that benzene is removed without consuming hydrogen or losing octane. Benzene alkylation, however, increases the gasoline boiling range somewhat due to the formation of heavier aromatics.
Alkylation with propylene produces a heavier material than alkylation with ethylene. Although propylene is more readily available than ethylene in most refineries, it is a valuable petrochemical and is also valuable as a feed to isobutane alkylation. Alkylation with ethylene produces lower-boiling aromatics than propylene alkylation, but requires recovering and potentially purifying ethylene, which adds cost to the process. - Extraction. Benzene for petrochemical use is recovered from light reformate using an aromatics extraction unit. Although petrochemical benzene can be an attractive product, there is significant investment required to recover benzene. This option is usually unattractive to refiners who make motor fuel and do not produce aromatics.
LP model
To help outline the options and provide guidance for evaluating benzene reduction, we set up a refinery linear program (LP) model for a typical refinery to evaluate how best to meet the new benzene regulations while also understanding the impact of blending ethanol and the lower VOC limits.
The LP model’s characteristics are for an example refinery processing 150,000 b/d of a mixture of Arab medium, Maya, and West Texas Intermediate crude. Gasoline production is about 87,000 b/d; about 30% is RFG (with 10 vol % ethanol) and 70% is conventional gasoline without ethanol. The grade mix is 11% premium and 89% regular for both RFG and conventional gasoline. All grades meet the summer southern-tier VOC requirements.
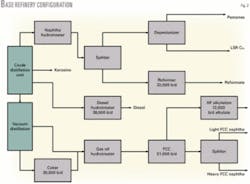
The base refinery configuration (Fig. 2) consists of a coker, heavy gas oil hydrotreater, FCC unit, HF alkylation unit, a distillate hydrotreater, and a catalytic reforming unit. The LP model includes two types of reforming units to assess the impact of reforming pressure-a continuous catalytic regeneration reforming unit or a fixed-bed reforming unit.
In the base case, reforming unit feed is deisohexanized. The light straight run (LSR) naphtha is depentanized for rvp blending flexibility when making RFG. Pentanes and LSR naphtha C6+ from the depentanizer are blended to gasoline with no additional processing. This example refinery is in naphtha balance; no naphtha is sold.
This base case configuration resulted in a gasoline pool containing about 2-vol % benzene, with either reforming unit.
Table 2 shows benzene contribution to the gasoline pool for the base case with the fixed bed and continuous catalytic reforming units.
Reforming unit operations can affect benzene contribution to the gasoline pool. In the base case, reforming unit feed is deisohexanized but there is no attempt to remove benzene precursors.
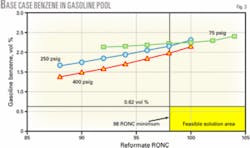
Fig. 3 shows gasoline pool benzene vs. reformate octane at several different reforming unit pressures. This shows the reforming unit operating severity and corresponding gasoline pool benzene level, which comes from the various streams blended. The minimum reformate research octane required to meet the gasoline requirements in the base case is 98 RONC.
In this example, RFG benzene is limited to 0.95 vol %. The benzene level in conventional gasoline is about 2.7 vol % because there is no reformate benzene control. The resulting benzene level in the overall gasoline pool (RFG plus conventional) of around 2.1 vol % is far greater than the 0.62 vol % benzene limit expected in the new regulations. Feasible solutions for making gasoline are therefore bounded by the minimum reformate octane and the maximum benzene allowed.
In Fig. 3, these solutions must fall within the area to the right of 98 RONC and below the horizontal line setting the benzene limit. In the base case, none of the operating curves fall within the feasible region; benzene control is necessary.
The simplest way to reduce benzene is to use the splitter to remove not only isohexane and lighter components but also benzene precursors from the reforming unit feed.
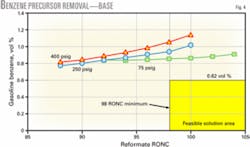
Fig. 4 shows the effect of removing benzene precursors from feed to the reforming unit operating at 400 psig, 250 psig, and 75 psig. For a gasoline pool target of 1-vol % benzene, the 75-psig reforming unit meets the target, the 250-psig reforming unit barely meets the target, and further processing is needed with the 400-psig reforming unit.
At the new 0.62-vol % benzene limit, prefractionation and blending the LSR naphtha into gasoline will not meet the benzene regulations at any operating pressure.
A review of the benzene contributions from the different streams highlights where to focus the additional processing options.
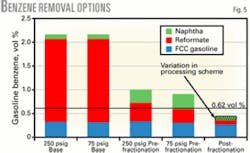
Fig. 5 shows the benzene levels with either a fixed bed or continuous catalytic reforming unit for the base case, with prefractionation to remove benzene precursors, and with postfractionation. Both cases blend to gasoline the light naphtha removed from the reforming unit feed.
The reformate contribution to gasoline benzene shrinks dramatically with reforming unit feed prefractionation.
Fig. 5 shows that prefractionation may allow the entire gasoline pool to meet a 1-vol % benzene limit with either reforming technology. Meeting the 0.62-vol % benzene limit, however, requires the refiner to address benzene in the light naphtha.
Fig. 5 also shows that removing all the benzene from the LSR naphtha is insufficient to meet the 0.62-vol % benzene limit with the fixed-bed reforming unit but may be sufficient with the lower-pressure continuous catalytic reforming unit.
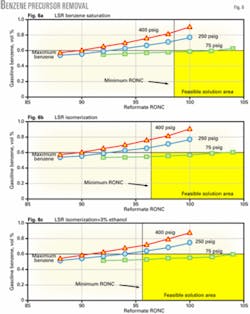
The simplest way to reduce benzene in light naphtha is to saturate it. This, however, will not meet the 0.62-vol % benzene target with the higher-pressure reforming units and barely meets the target with the continuous catalytic reforming unit (Fig. 6a). Whether light naphtha benzene saturation meets the gasoline benzene limit depends on the benzene contribution to the gasoline pool from other blending components, especially FCC naphtha, as well as the operating pressure of the continuous catalytic reforming unit.
Providing additional compliance margin while achieving the 0.62-vol % benzene limit requires adding other nonaromatic (nonreformate) octane, which will shift the required reforming unit octane to the left and move the resulting pool benzene downward on the benzene-octane relationship.
One way of doing this is to add nonaromatic octane by isomerizing the light naphtha, which will also saturate benzene. This processing step allows the reforming unit to operate at lower severity, resulting in a greater compliance margin to attain the benzene regulation.
Fig. 6b shows that isomerization of light naphtha reduces the minimum reformate octane required to meet the gasoline octane, thereby providing additional compliance margin.
In the base case, RFG contains 10-vol % ethanol, which is about 3 vol % of the entire gasoline pool for a refiner producing 30% RFG. With the new RFS requirements, it is likely that ethanol will also be blended into conventional gasoline.
One option investigated in this study was to determine whether it would be easier to meet the gasoline benzene target if more ethanol is blended. In this case, 10% ethanol is blended into just less than half of the conventional gasoline pool and the amount of total ethanol blended is doubled to 6 vol % of the entire pool.
Fig. 6c shows that increasing the volume of ethanol blended reduces the reforming unit octane needed to make gasoline and increases the solution area that meets the benzene limit. Only the continuous catalytic reforming unit, however, meets the benzene limit. Blending more ethanol provides an operating margin for low-pressure reforming units but will not enable fixed-bed reforming units to meet the 0.62-vol % benzene limit with prefractionation.
Meeting the 0.62-vol % benzene limit with higher-pressure reforming units requires another strategy. Postfractionation provides better benzene control because the control point is moved downstream of the reforming unit. Postfractionation, however, requires investment in a reformate splitter and processing to remove benzene.
Postfractionation (Fig. 1b) also offers somewhat greater gasoline blending flexibility than prefractionation because a high octane, low-rvp blendstock is produced in the reformate splitter.
In addition, postfractionation removes reforming unit operating severity and pressure as concerns. Finally, because postfractionation reduces reformate benzene to low levels, there is a greater compliance margin in meeting the new benzene limits. The gasoline benzene levels achieved with postfractionation are relatively insensitive to the type of reforming unit or the processing technology chosen to remove benzene.
Fig. 5 shows benzene levels for the various options; postfractionation achieves lower benzene than prefractionation. The variation in benzene for postfractionation results from the different processing routes used to reduce benzene. Substantially more benzene reduction will require treating FCC naphtha.
The benzene range in Fig. 7 brackets the level of gasoline benzene achieved via postfractionation using the various benzene reduction options: benzene saturation, benzene alkylation, benzene extraction, or light reformate isomerization with isomerization of LSR naphtha.
Hydrogen consumption
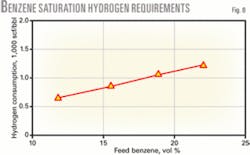
Hydrogen is needed to saturate benzene; therefore, the refinery hydrogen balance is important when choosing a benzene reduction strategy. Hydrogen consumption for benzene reduction is primarily dependent on the benzene concentration in the benzene removal unit feed stream.
Fig. 8 shows hydrogen required to saturate benzene as a function of feed benzene content. When benzene saturation occurs with isomerization of light paraffins, a small amount of additional hydrogen is needed for the isomerization reactions.
Benzene alkylation and extraction do not require hydrogen and are attractive options for benzene reduction. Refiners that produce aromatics already remove much, if not all, of the benzene from their gasoline.
For refiners making motor fuel, benzene extraction will require investment in infrastructure to store and process gasoline streams with high benzene levels. This investment is likely to deter many motor fuel refiners from considering this option.
Benzene alkylation may be an attractive option for dealing with benzene in light reformate; however, benzene alkylation depends on the availability of olefins to alkylate benzene, the steps necessary to recover and clean up these olefins for alkylation, and the effect of benzene alkylation on gasoline distillation.
Recommendations
Refiners with low-pressure reforming units may be able to use prefractionation to meet the new gasoline benzene regulations. Some additional operating flexibility is possible with additional nonaromatic octane that can help reduce reforming unit severity needed to meet gasoline pool octane. This additional octane can come from isomerizing the light naphtha produced when removing benzene and benzene precursors from the feed to the reforming unit, and by blending more ethanol into the gasoline pool or purchasing other high-octane blendstocks.
Refiners with higher-pressure reforming units are less likely to meet the new benzene limits with prefractionation and may need to use postfractionation.
Postfractionation offers better benzene control and provides additional operating and gasoline blending flexibility. Benzene levels achieved in postfractionation are relatively insensitive to the method used to handle the benzene-containing light reformate stream.
Saturating benzene in the light reformate or isomerizing the light reformate in conjunction with the light naphtha are options that will help refiners meet the benzene target and result in some increase in gasoline pool volume, but will require hydrogen.
Benzene extraction is an attractive solution to refiners with access to extraction units and the infrastructure to handle concentrated benzene streams. Benzene alkylation, while offering an attractive solution that does not require hydrogen or significantly affect gasoline octane, may affect gasoline distillation; this solution depends on olefin availability.
Reference
- Keesom, W.H., Bozzano, U., and Raman, P., “Benzene Reduction Alternatives,” presented to the 1991 National Petrochemical & Refiners Association Annual Meeting, San Antonio, Mar. 17-19, 1991.
Based on a presentation to the National Petrochemical & Refiners Association Annual Meeting, Salt Lake City, Mar. 19-21, 2006.
The authors
Jill Meister (Jill.Meister@ UOP.com) is the senior alkylation and oxygenates specialist of the gasoline development department in UOP LLC’s process technology and equipment business group, Des Plaines, Ill. She started her career with UOP in 1988. Meister’s background includes roles in research and development, field operating services, UOP’s modular systems group, and as a gasoline business technology manager. She holds a BS in chemical engineering from Michigan Technological University, Houghton, and is a member of the school’s presidential council of alumnae.
Tom Crowe is a senior consultant in UOP LLC’s optimization services group, responsible for refinery configuration and profitability analysis. Before joining the optimization services group, he held various positions at UOP relating to optimization, naphtha reforming, isomerization, and process engineering. Crowe holds a BS in chemical engineering from the University of Illinois, Urbana, and an MS in electrical engineering from the Illinois Institute of Technology.
William Keesom is a senior consultant in UOP LLC’s optimization services group where he helps refiners evaluate refinery configurations for improved profitability or for meeting new fuel or market requirements. For more than 15 years he has consulted on meeting clean fuels requirements. Keesom holds an MS in chemical engineering from the University of California, Berkeley, and a Masters of Management from Northwestern University.
Margaret Stine is the director of marketing in UOP LLC’s process technology and equipment strategic business unit. She has held various positions at UOP in research and development, the technical services area, and engineering and product line management. Stine holds a BS from Vanderbilt University and an MBA from the University of Chicago.