Scale-up strategy applied to solid-acid alkylation process
Mitrajit Mukherjee, James Nehlsen, Sankaran Sundaresan, G. Dan Suciu, John Dixon - Exelus Inc., Livingston, NJ
A new isoparaffin alkylation process using a solid-acid catalyst has recently been scaled up and proven in a pilot plant using a strategy outlined in this article.
This new process is predicated on two key components: a unique, engineered solid-acid catalyst1 and a simple fixed-bed reactor that is designed to enhance the performance of the solid-acid catalyst.2 The engineered catalytic system has a highly structured pore architecture, which enhances transport processes within the catalytic system leading to better system stability.
The larger pores provide efficient access and quick diffusion of reagents to the microporous system and the smaller pores provide the surface area required to contain the optimized acid sites. The integration of catalyst science and reaction engineering allows catalyst lifetimes that are an order of magnitude longer than most solid-acid catalysts. Long catalyst life, in turn, permits efficient and inexpensive process and reactor schemes.
Catalyst performance and stability were demonstrated on a pilot-scale reactor that closely matched the commercial reactor design. Tests showed that product quality and catalyst lifetime is identical in the bench scale and pilot-scale reactors when operated at the same temperature, feed composition, and olefin space velocity.
Applying this scale-up strategy and a fully integrated catalyst development technique resulted in a high product octane rating and a simple process operation.
Microscale reactors were used for the initial catalyst screening and to develop mathematical models of the reaction kinetics. Bench-scale reactors provided the data to optimize catalyst performance and to design the reactor. These tests also helped determine the effects of various process parameters.
Finally, a pilot unit was used to verify all of the results with conditions identical to the commercial reactor. The pilot unit was designed to match all of the important transport and reaction properties of the commercial reactor.
Integrated approach
Moving a new refinery process from inception to commercialization requires many phases of development. Catalytic processes, in particular, require development at several scales in order to first create and optimize the catalyst, then to optimize the reactor design and process conditions, and finally to demonstrate the performance on an appropriate pilot scale.
To further complicate the development, these stages are not independent because the catalyst characteristics, reactor design, and process conditions all contribute to the overall process design and economics.
When developing the step-out alkylation catalyst, we used a fully integrated approach to process design to achieve the best possible process performance. The underlying principle of this approach is that the catalyst, reactor, and supporting unit operations are all parts of a single process that should be designed simultaneously rather than linearly, and always considering the practical application and cost of the process.
This approach can lead to significant improvements in process economics and shorten the process development time, with its own economic benefits.
Our goal was to develop a simple swing fixed-bed reactor process using an environmentally safe solid-acid catalyst. We wanted to use a fixed-bed reactor for two reasons: to simplify process scale up and to keep capital costs and operating expenses as low as possible.
In addition to being a 1-3 mm diameter particle to function in a fixed-bed reactor, the solid-acid catalyst had to be robust. Consequently, the ideas of replacing the conventional liquid acids with a liquid acid supported on solid particles or co-feeding toxic halogens to a solid-acid catalyst to maintain its activity were rejected right at the outset. Only a chemically stable, solid acid would meet the requirements.
Our development approach was first to understand the catalysis of the alkylation reaction by systematically studying the effects of physical and chemical parameters of the solid-acid catalyst on product distribution and catalyst deactivation characteristics in a microreactor.
We developed a robust kinetic model from data obtained in the microreactor. The model was used to engineer the solid-acid catalyst to provide optimal performance in terms of catalyst life and product octane. The model was also used to develop a viable fixed-bed reactor design.
We then studied the optimized solid-acid catalyst in a bench-scale reactor under commercially relevant conditions. Process conditions for operating the alkylation unit along with the catalyst design and reactor configuration were fine-tuned with the bench-scale reactor data.
Finally, we used the optimized catalyst and chosen reactor configuration to design and run a pilot plant. The product octane was more than 98 RON using a methyl tertiary butyl ether raffinate feed in the pilot plant over multiple cycles of alkylation followed by regeneration, confirming the viability of the solid-acid alkylation technology.
Microreactor studies
We conducted initial catalyst screening experiments in a once-through microreactor using 0.5-1.0 g of catalyst. To achieve acceptable catalyst lifetimes, we conducted screening tests in the microreactor at feed isoparaffin-to-olefin (I:O) ratios of 100-200 to suppress the olefin oligomerization reaction. We evaluated several different catalyst formulations encompassing a wide range of acid functions, number of acid sites, and acid strengths as part of this study.
Through a systematic study, we were able to identify an optimal window of design parameter values that were then used to develop the new solid-acid catalyst.
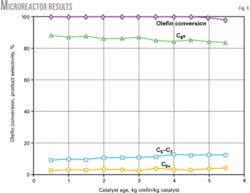
Fig. 1 shows typical data from the microreactor.
Mathematical modeling
The microscale studies provided information for initial correlations of catalyst performance with reaction conditions. Analyzing the rate data obtained from the microreactor allowed us to develop a robust model that can predict product octane as well as catalyst lifetimes for a large range of reaction conditions.
This model accounts for the effect of intraparticle diffusion, which is substantial, and can accurately predict the rate of catalyst deactivation caused by the formation of heavy oligomers. The model was validated with experimental data.
The analyses also revealed that the rate constant for the undesired secondary (oligomerization) reaction is two orders of magnitude higher than the rate constant for the primary alkylation reaction.3 To reduce substantially the rate of oligomerization, therefore, the olefin concentration near the catalytically active sites must be two orders of magnitude lower than the isoparaffin concentration.
Using the reaction model, we simulated several scenarios of pore size and acid site distribution within the catalyst particle. The configuration that gave the longest life with the highest-octane product was chosen and catalyst synthesized accordingly.
Bench-scale studies
Once the basis for the catalyst and reactor design was established, larger bench-scale studies were initiated.4
To maintain a high I:O ratio (about 400) in the reactor while using commercial grade feedstocks (I:O ratio of 10-15), we used a fixed-bed recycle reactor to conduct most of the research work. Typically 5-10 g of catalyst were used for catalyst testing with a feed rate of 50-100 g/hr.
The main purpose of the research was to:
- Fine-tune the solid-acid catalyst design.
- Generate data to enable design of viable fixed-bed reactors.
- Study the effect of temperature, feed composition, and olefin space velocity on catalyst stability and product quality.
The bench-scale results for the alkylation process revealed that the solid-acid catalyst produces alkylate with a high octane rating over a wide range of operating temperatures (60-90° C.), olefin space velocities (0.1-0.5 hr-1), and feed compositions (I:O ratios of 10-15). Its robustness minimizes the requirements for feed pretreatment.
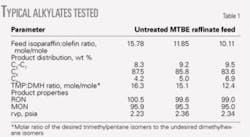
Table 1 summarizes results from the bench-scale testing of some recently synthesized catalysts. These catalysts are an improved version of the one used for pilot-plant testing. The octane values and Reid vapor pressures were computed with gas chromatography (GC) product analysis.5 Typical ASTM engine test octane values tend to be higher than those computed with the GC method.
The trimethylpentane-to-dimethylhexane (TMP:DMH) molar ratio in the alkylate product indicates the relative degree to which the primary reaction is isobutane alkylation or olefin polymerization. Dimethylhexanes arise from olefin dimerization followed by hydride transfer; trimethylpentane formation largely depends upon paraffin-olefin combination to form a carbonium ion species followed by hydride abstraction from isobutane.
A higher the TMP:DMH ratio indicates a greater effect of alkylation reactions, in contrast to olefin polymerization. With HF or with H2SO4, the TMP:DMH ratio is 3-6. These values are also typical of most solid-acid catalysts reported in literature.
The high TMP:DMH ratio for the new catalyst is the reason for the long catalyst lifetimes and high product octane; the olefin polymerization reaction have been suppressed and the alkylation reaction rates are increased. The long catalyst lifetimes enable the use of a simple fixed-bed reactor.
Designing the pilot unit
After the catalyst and reactor design were complete, we needed to test the catalyst on a pilot scale. Pilot testing is essential to:
- Generate enough alkylate to measure product octane with an engine test.
- Demonstrate catalyst stability.
- Develop critical data to allow successful scale-up to a commercial-scale plant.
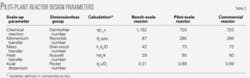
In anticipation of the commercial-reactor design, we chose a pilot-plant configuration that included multi-staged olefin feed with product recycle. We carefully designed the pilot unit by maintaining the same scale-up parameters in the pilot unit as in the commercial-scale reactor.
Interphase heat and mass-transfer effects are important for liquid acid alkylation reactors but do not play a major role in the solid-acid alkylation process.
Table 2 compares the critical scale-up parameters for the two reactors (see accompanying box: nomenclature for dimensionless numbers). Table 2 shows that the scale-up parameters are matched, which should enable a seamless scale-up from the pilot unit to a commercial-scale plant.
The critical parameters in the alkylation process are reaction temperature, feed composition, olefin space velocity, and bed I:O ratio. As long as these parameters remain the same, catalyst performance is not a strong function of the transport parameters (Table 3). In other words, scale-up of the new process from bench scale to a commercial plant is a comparatively simple exercise.
null
Pilot-plant performance
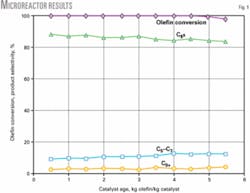
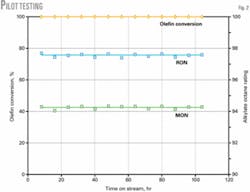
Catalyst performance was validated in the pilot unit for an extended time. Catalyst stability, product octane, and alkylate yield were proven in the pilot-scale reactor (Fig. 2).
We confirmed catalyst stability by measuring the catalyst performance in multiple cycles of alkylation and regeneration using hydrogen. Alkylation cycles were 8-12 hr followed by a 2-hr regeneration cycle. The time on stream reported here refers to the cumulative time of the alkylation cycles only.
Fig. 2 shows that there is no activity loss over repeated cycles of alkylation and regeneration as measured by olefin conversion, product octane, or alkylate yield. The product octane is significantly higher than that obtained with liquid-acid processes under similar operating conditions and feedstocks. The olefin feed, which contained more than 3,500 ppm of diolefins, 700 ppm of oxygenates, and 0.5 ppm of acetonitrile, was untreated for these tests.
Product quality was studied as a function of process conditions, olefin feed concentration, and olefin type. Product octane is only moderately sensitive to olefin space velocity but decreases 1 octane point for a 15° C. rise in temperature.
Alkylate yields are typically 2.15-2.2 weight alkylate/weight olefin consumed for the range of process conditions studied. There is no difference in catalyst performance between 1-butene and 2-butene feeds, which is in stark contrast to liquid-acid processes.
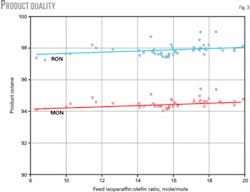
Fig. 3 shows that product quality is essentially unaffected by feed olefin concentration in the range of commercial interest (feed I:O ratios of 8-20). Isobutylene has a slightly negative influence on product quality. An FCC olefin mix containing 30% isobutylene produces a 3 octane-point loss compared to an MTBE raffinate feed.
Commercial process scheme
Scaling up the process from a pilot-plant reactor to a commercial-scale reactor is a relatively straightforward process. Because the hydrodynamic, transport, and chemical reaction parameters remain the same, there is little else that is different in these two reactors except for the physical size of the vessel.
One added advantage of the isoparaffin alkylation process is the low heat of reaction, which minimizes the need for large heat-transfer surfaces to be integrated within the reactor volume.
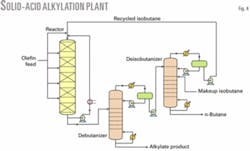
The new process scheme consists of two swing-bed reactors and a separation section, which minimizes capital costs.6 The separation section resembles those used in typical liquid-acid alkylation plants and, as such, can be easily retrofitted into existing liquid acid alkylation plants, catalytic polymerization units, and idle MTBE units.
The revamp of a catalytic polymerization unit is simple because two or more multistage fixed-bed reactors are already in place. The primary changes entail adding some optional feed-treating equipment and installing a small reactor recycle pump and a recycle hydrogen compressor for the catalyst reactivation section. Minimal modifications to the existing distillation units are required.
Fig. 4 shows a revamp configuration using multiple olefin feed stages with product recycle. The reactor is operated in a swing mode such that one reactor is on alkylation while the other is on catalyst regeneration.
Alkylation cycle lengths are designed to be 12-24 hr to simplify reactor operation. Every 12-24 hr, the alkylation reactor is removed from service and the solid-acid catalyst is regenerated with excess hydrogen under mild conditions. The regeneration step lasts 2 hr. Due to the small amount of coke build up on the catalyst surface, hydrogen consumption is minimized.
Acknowledgment
This work was partially funded through grants from the US Department of Energy and the National Science Foundation.
References
1. Mukherjee, M., and Sundaresan, S., “ExSact-A Step-out Isoparaffin Alkylation Technology, Part 1. Catalyst Development,” World Refining, January/February 2005, pp. 28-31.
2. Mukherjee, M. and Sundaresan S., “ExSact-A Step-out Isoparaffin Alkylation Technology, Part 2. Reactor Development,” World Refining, March 2005, pp. 22-26.
3. Simpson, M.F., Wei, J., and Sundaresan, S., “Kinetic Analysis of Isobutane/Butene Alkylation over Ultrastable H-Y Zeolite,” Industrial Engineering and Chemistry Research, 1996, No. 35, pp. 3861-73.
4. Mukherjee, M., and Suciu, G.D., “ExSact-A Step-out Isoparaffin Alkylation Technology, Part 4. Solid-Acid Catalyst Optimization,” World Refining, June 2005, pp. 28-31.
5. Hutson, T., and Logan, R., “Estimate Alkylate Yield and Quality,” Hydrocarbon Processing, September 1975, pp. 107-10.
6. Mukherjee, M. and Porcelli, R., “ExSact-A Step-out Isoparaffin Alkylation Technology, Part 3. Process Economic Evaluation,” World Refining, April/May 2005, pp. 32-35.
The authors
Mitrajit Mukherjee ([email protected]) is the founder and president of Exelus Inc., Livingston, NJ. He has spent most of his 17-year career developing viable solid-acid catalyst solutions for isoparaffin alkylation. Before founding Exelus, he held positions at Catalytica and ABB Lummus Global. Mukherjee holds a BS in chemical engineering from the Indian Institute of Technology and an MS from the Southern Illinois University. He is a member of ACS and AIChE
James Nehlsen ([email protected])is a senior research engineer at Exelus Inc. specializing in the development of new catalytic processes. He holds a PhD in chemical engineering from Princeton University where he developed new desulfurization technologies. Nehlsen is a member of ACS and AIChE.
Sankaran Sundaresan is director of research and development at Exelus and also a professor of chemical engineering at Princeton University. He has authored more than 100 journal publications in many fields, including solid-acid alkylation. He holds a PhD from the University of Houston. Sundaresan is an editor of the AIChE Journal. He is a member of ACS and AIChE.
G. Dan Suciu is a senior research advisor at Exelus Inc. He was previously vice-president of research and development at ABB Lummus Global. He is the inventor of many first-of-a-kind technologies including fluidized bed maleic anhydride and liquid phase ethylbenzene processes. Suciu holds numerous patents and recently edited the book “Thermal and Catalytic Processes in Petroleum Refining.” He holds a PhD from Polytechnica University, Buucharest, Romania. He is a member of ACS
John Dixon is a senior research specialist at Exelus Inc. He has more than 40 years’ experience in catalyst evaluation with companies including Mobil Corp. and Exxon Corp., including 16 years working with alkylation technologies. Dixon also holds a patent for synthesizing high viscosity lubricants.