Correlation measures properties of brominated petchems
A new correlation predicts the solubility and Henry’s law constant for a wide range of brominated petrochemicals in water. This new correlation provides reliable solubility values down to very low concentrations for monobrominated alkanes in water.
It is based on the boiling-point temperature of the brominated compound. The correlation shows favorable agreement with experimental data and is useful to engineers for health, safety, and environmental studies.
Water solubility
Estimating the solubility of brominated compounds in water is very important. This importance will increase due to future health, safety, and environmental issues.
For human exposure to substances in air, for example, OSHA has established the threshold limit value for bromoehtane in air at 5 ppm (vol). A concentration of only 0.000001 mole fraction of bromoethane in water leads to a value of 403.3 ppm (vol) in air at the air-water interface.
For safety reasons, the lower explosion limit for bromoethane in air is 6.7%.1 A concentration of only 0.0002 mole fraction of bromoethane in water leads to a value of 8.1% in air at the air-water interface.
Environmental example: Consider a spill of bromoethane in water. At saturation, the solubility of bromoethane in water is about 0.0015 mole fraction, or about 9,000 ppm (wt).2 This water concentration leads to an air concentration of 60.5% at the air-water interface. This concentration exceeds the threshold limit value and the lower explosion limit.
Correlation
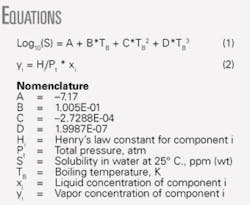
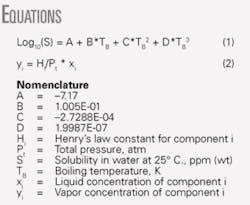
In an earlier work, we correlated water solubility for hydrocarbons and other chemicals as a function of the compound’s boiling point.1 We determined that the boiling-point method was also applicable for correlating the water solubility of monobrominated alkanes (see Equation box).
Click here to view Properties of Bromminated Compounds in pdf.
Equation 1 applies to compounds with boiling temperatures of 280-590 K.
We determined the correlation coefficients (A, B, C, and D) from regressing available data.1-10 We used extensively the compilations by Howard and Meylan;3 Mackay, Shiu, and Ma;4 Vershueren;5 Yalkowsky;6 and Yaws.1 7
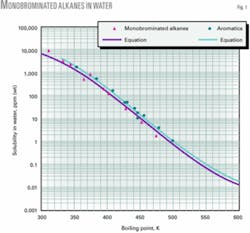
Fig. 1 shows the solubility vs. boiling temperature for monobrominated alkanes. It also shows that the curve for aromatics is similar to that of monobrominated alkanes.
Henry’s law constant
Table 1 shows the results for water solubility and Henry’s law constant. The results for Henry’s law constants are based on the water solubility and vapor pressure at ambient conditions using appropriate thermodynamic relationships.
Example 1
A chemical spill of 1-bromopentane occurs into a body of water at ambient conditions (25° C., 1 atm)-estimate the concentration of 1-bromopentane in water at saturation.
Substitution of the coefficients and boiling point into Equation 1 yields:
Log10(S) = -7.17 + 1.005E-01*402.74 - 2.7288E-04*(402.74)2 + 1.9987E-07*(402.74)3
Log10(S) = 2.10072
S = 126.1 ppm (wt)
Example 2
A chemical spill of 2-bromobutane occurs into a body of water at ambient conditions. The concentration in the liquid at the water’s surface is 0.0001 mole fraction-estimate the concentration of 2-bromobutane in the air at the air-water interface.
From thermodynamics at low pressure, Equation 2 gives the vapor concentration.
Substituting Henry’s law constant, total pressure, and the liquid concentration into Equation 2 yields:
yi = 785.82/1 * 0.0001 = 0.07852
yi = 7.85 mole %.
Acknowledgment
The Texas Hazardous Waste Research Center at Lamar University, Beaumont, Tex., provided partial support for this article. ✦
References
1. Yaws, C.L., Chemical properties handbook, New York: McGraw-Hill Cos., 1999.
2. Horvath, A.L., Halogenated hydrocarbons: Solubility-miscibility with water, New York: Marcel Dekker Inc., 1982.
3. Howard, P.H., and Meylan, W.M., ed., Handbook of physical properties of organic chemicals, Boca Raton, Fla.: CRC Press, 1997.
4. Mackay, D., Shiu, W.-Y., and Ma, K.-C., Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals, Vols. 1-5, New York: Lewis Publishers Inc., 1992-97.
5. Vershueren, K., Handbook of environmental data on organic chemicals, 3rd and 4th eds., New York: Van Nostrand Reinhold, 1996, 2001.
6. Yalkowsky, S.H., Aquasol database, University of Arizona, Tucson, 1990-2002.
7. Yaws, C.L., Yaws handbook of thermodynamic and physical properties of chemical compounds, electronic database, www.knovel.com, Norwich, NY: Knovel Corp., 2003.
8. CRC handbook of chemistry and physics, 75th-84th eds., Boca Raton, Fla.: CRC Press Inc., 1994-2004.
9. Handbook of environmental data on organic chemicals, 4th ed., New York: John Wiley & Sons, 2001.
10. Horvath, A.L., and Getzen, F.W., Journal of Physical Chem. Ref. Data, Vol. 28 (1999), No. 3, pp. 649-777.
The authors
Carl L. Yaws ([email protected]) is professor of chemical engineering at Lamar University, Beaumont, Tex. His research interests include technology development, thermodynamic and transport property data, environmental engineering, and process simulation. Yaws holds BS in chemical engineering from Texas A&I University, Kingsville, and an MS and PhD in chemical engineering from the University of Houston. He is a registered professional engineer in Texas.
Prasad K. Narasimhan is graduate student in chemical engineering at Lamar University. His research interests include thermodynamics, environmental engineering, and process simulation. He holds a BS in chemical engineering from Siddaganga Institute of Technology, India.
Ralph W. Pike is Paul M. Horton Professor of Chemical Engineering and the director of the Minerals Processing Institute at Louisiana State University, Baton Rouge. His research interests include process optimization, fluid dynamics, reactor design, ecological systems, and pollution prevention. Pike holds a BS and PhD from Georgia Institute of Technology, Atlanta. He is a registered professional engineer in Louisiana and Texas.