Simple cubic EOS predicts properties of pure hydrocarbons, mixtures
A new, simple form of a cubic equation of state (EOS) has been developed in a recent study. A comparison to experimental data and other cubic equations of state show that the new EOS is more accurate.
Oil and gas industry engineers require a simple, extensive, and more reliable EOS that accurately calculates the properties of pure hydrocarbons and hydrocarbon mixtures for crude processing, gas processing, design, and operations.1-3
The application of equations of state as essential tools for calculating phase equilibria has been the subject of numerous studies. From the many equations of state proposed in the literature, cubic equations of state have proved to correlate well the phase equilibria of highly nonideal systems.
Most of the cubic equations of state are a modified, generalized van der Waals equation (OGJ, July 12, 1993, p. 108; June 18, 1973, p. 82).4-7 Process engineers prefer to use a simple cubic EOS.
This article discusses the application of the new equation for pure hydrocarbon and hydrocarbon mixtures. The calculated results obtained from the new EOS are compared with those using the Redlich-Kwong (RK),5 Soave-Redlich-Kwong (SRK),6 and Peng-Robinson (PR)7 equations of state.
New equation of state
We developed the new cubic EOS to provide a precise representation of the pure and mixed fluids calculations of interest to the oil and gas industry (Equations 1-5). Because the RK, SRK, and PR are known as the best cubic equations of state for pure hydrocarbon and hydrocarbon mixtures, we compared them to show the advantage of the new equations.
Equation 5 calculates the density and equilibrium states for pure hydrocarbons and binary mixtures, as well as a selected oil mixture fluid.
The volumetric property calculations at different temperatures and pressures were compared with derived results from PR, SRK, and RK equations of state.
The new EOS can be used for calculating pressure-volume-temperature and vapor-liquid equilibrium for pure hydrocarbons and hydrocarbon mixtures.
Calculations
Practicing engineers need volumetric properties in various stages of design and process operations. We therefore applied the new equation to calculate the densities of light and heavy pure hydrocarbons from 50 to 5,000 bar. All of the experimental data of pure hydrocarbons are obtained from Vargaftik.8
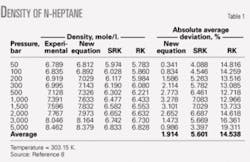
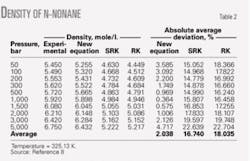
Tables 1 and 2 compare the densities of n-heptane and n-nonane obtained from the new, RK, SRK, and PR equations of state. These tables show that the new equation is much more accurate than the RK, SRK, and PR correlations.
Absolute errors for densities at different pressures from the new equation, the SRK, and the RK equations of state are, respectively, 1.914%, 5.601%, and 14.538% for n-heptane; 2.038%, 16.74%, and 18.035% for n-nonane; 11.372%, 28.199%, and 139.795% for n-tridecane. For n-triocsane, the errors were 5.913% and 48.206% for the new and RK equations, respectively.
The PR equation cannot be used to predict densities of pure heavy hydrocarbons.
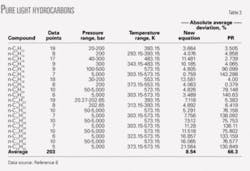
Table 3 shows the results of calculated densities from the new and PR correlations. The percent error for the compounds C5-C20 were 8.54% and 66.3%, respectively.
We also performed pressure-volume-temperature and vapor-liquid equilibrium calculations using the new EOS to obtain the densities of binary and ternary mixtures. Equations 6 and 7 show the application of the common and widely used mixing rule for a and b. The kij parameter is assumed to be zero for all the calculations.
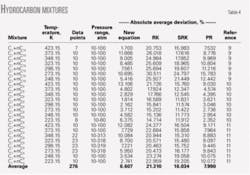
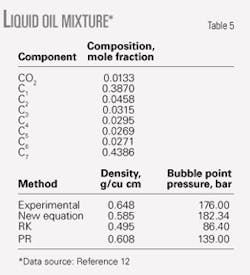
Tables 4 and 5 show the calculated results for binary mixtures and for an oil mixture. The new EOS predicts the densities of mixtures more accurately than the RK and PR equations of state.
The new correlation presented in this article is a simple two-constant parameter cubic equation. It is relatively accurate and is a suitable and efficient tool for chemical engineering calculations.
The equation developed is accurate enough to replace other equations of state for the pressure-volume-temperature calculations for oil and gas mixtures.✦
References
1. Nasrifar, K., and Moshfeghian, M., “Application of an Improved Equation of State to Reservoir Fluids: Computation of Minimum Miscibility Pressure,” J. Pet. Sci. Eng., Vol. 42 (2004), pp. 223-34.
2. Erdogmus, M., and Adewumi, M.A., “A Modified Equation of State for Gas-Condensate Systems,” presented to the SPE conference, Oct. 17-19, 2000, Morgantown, W.Va.
3. Nasrifar, K., and Moshfeghian, M., “Vapor-liquid Equilibria of LNG and Gas Condensate Mixtures by Nasrifar-Moshfeghian Equation of State,” Fluid Phase Equilib., Vol. 200 (2002), pp. 203-16.
4. Van der Waals, Doctoral Dissertation, Leiden, The Netherlands, 1873.
5. Redlich, O., and Kwong, J.N.S., “On the thermodynamics of solutions,” Chem. Review, Vol. 44 (1949), pp. 233-44.
6. Soave, G., “Equilibrium constants from a modified Redlich-Kwong equation of state,” Chem. Eng. Science, Vol. 27 (1972), pp. 1197-1203.
7. Peng, D.Y., and Robinson, D.B., “A new two constant equation of state,” Ind. Eng. Chem. Fundamentals, Vol. 15 (1976), pp. 59-64.
8. Vargaftik, N.B., “Handbook of Physical Properties of Liquids and Gases (Pure Substances and Mixtures),” 2nd Ed., Hemisphere Publications, Washington, DC, 1975.
9. Beaudoin, J.N., and Kohn, J.P., “Multiphase and Volumetric Equilibria of Methane-n-Decane Binary System at Temperatures between -36 and 150 C,” J. Chem. Eng. Data, Vol. 12 (1967), pp. 189-91.
10. Shima, J., and Kohn, J.P., “Multiphase and Volumetric Equilibria of Methane-n-Hexane Binary System at Temperatures between -1.10 and 150 C,” J. Chem. Eng. Data, Vol. 7 (1962), pp. 3-8.
11. Shipman, L.M., and Kohn, J.P., “Heterogeneous Phase and Volumetric Equilibrium in the Methane-n-Nonane System,” J. Chem. Eng. Data, Vol. 11 (1966), pp. 176-80.
12. Simenat, R., “Phase Behavior of CO2-Reservoir Oil System,” SPE Journal, Vol. 18 (1978), No. 1.
The authors
Mohsen Edalat (iranedalat@ yahoo.com) is a professor of chemical engineering at the University of Tehran, Iran. He holds a BSc in chemical engineering from the University of Tehran and an MSc and PhD in chemical engineering from the University of Illinois.
G.R. Pazuki (ghpazuki@ yahoo.com) is currently a PhD student in chemical engineering at the Malek e Ashtar University of Technology, Tehran, Iran. He holds a BSc in petroleum process engineering from Sharif University of Technology, Iran, and an MSc in chemical engineering from Tarbiat Modarres University, Iran.
A. Dashtizadeh is in charge of the engineering and design section of South Pars Gas Co., Tehran, Iran. He holds BSc and MSc degrees in chemical engineering from the Sharif University of Technology, Iran.