Study compares C6+ characterization methods for natural gas phase envelopes
A recent study applied different C6+ characterization techniques to generate a phase envelope for natural gases and, using these techniques and a single dewpoint measurement, generated a relatively correct phase envelope.
Using the single dewpoint measurement, we determined the appropriate component or cut distribution for C6+ and evaluated the accuracy of each method for two case studies covering rich and lean natural gases.
We evaluated the effect of four C6+ characterization techniques for generating phase envelopes for natural gases. With these techniques, a single dewpoint measurement was used to generate a relatively correct phase envelope.
Using the single dewpoint measurement, we determined the appropriate component or cut distribution for C6+ by adjusting the C6+ properties such as molecular weight, critical temperature, etc.
The exponential decay, or logarithmic, distribution of C6+ will improve the prediction accuracy of potential hydrocarbon liquid content estimations by use of a C6+ molecular weight to match a single dewpoint. This approach is internally consistent and results in generation of relatively accurate phase behavior.
For a sound process design or operation, we suggest using at least one experimental saturation measurement such as a dewpoint or bubblepoint near the potential operating conditions to characterize the heavy ends. Once the characterization is established, one should use additional experimental measurements to verify the accuracy and validity of the tuning technique.
Phase diagrams
In facilities operations, the understanding of where the process is on a phase diagram can often help the engineer and operator avoid design and operating mistakes. The oil and gas industry is full of many “war stories” about “phase diagram disasters.” Most instances are never related back to the phase diagram misunderstanding.
In one well-documented but poorly published case, a dry gas pipeline that was pigged flooded miles of sandy beach. In another case, thousands of kilowatts of compression power were installed to maintain the pressure of a reservoir above the dewpoint when in fact the reservoir was at a temperature above the cricondentherm.
Equipment manufacturers and gas purchasers have often made specifications for “superheat” or dewpoint that have not been met and led to upset customers or millions of dollars of lawsuits.
One of the first issues that a gas plant or gas production facility engineer must resolve should be: Where is the process operating with respect to the phase diagram? A general knowledge, if not a detailed knowledge, will allow the design engineer and the facilities operator to make intelligent decisions that significantly affect the profitability of a gas production plant.
The phase envelope has many applications in petroleum production and process design ranging from reservoir simulation, pumping liquids, transportation of natural gas by pipeline or in LNG form, ethane and heavier recovery, refrigeration processes, and operation near the critical point or in the supercritical region. A sound process design requires a good knowledge of the phase envelope.1
For a petroleum fluid, one can construct a phase envelope using experimental measurements of a series of bubblepoints and dewpoints. This, however, is very time consuming and expensive. With a little care and experience, an accurate phase envelope can be constructed with an equation of state based on a limited number of experimental measurements.1 2
In many cases, real fluid mixtures consist of dozens, if not hundreds, of components. Attempting to consider all of the components present in such a mixture is impractical because experimental determination of each component may not be feasible or possible.
More importantly, the vapor-liquid equilibrium calculations for systems containing many components would be time consuming. The required calculation time increases exponentially with the number of components in the mixture. To overcome this difficulty, the components heavier than hexane and their derivatives are normally lumped together as one or more pseudocomponents called the heavy ends, C6+,or C7+.1
There are many references that describe techniques for characterizing C6+ fractions.1-6 The C6+ fraction is frequently the determining factor in defining the dewpoint of the gas.
Liquid formation in a natural gas pipeline changes the line pressure drop and flow characteristics. A seemingly inconsequential amount of liquid can cause significant changes in line pressure drop or capacity (OGJ, Feb. 4, 2002, p. 56).7
This article presents and briefly describes several methods of heavy-ends characterization, checks the accuracy of each method, and presents tips to improve the accuracy of each method. We also included a recent method for characterizing heavy ends described by Starling to generate a more accurate phase envelope for natural gases.8
This technique uses a single experimentally measured dewpoint and assumes a logarithmic distribution, presented by Katz,9 of nC6H14 to nC17H36 for heavy ends. This technique has been verified by matching the potential hydrocarbon liquid content (PHLC) of the gas. The PHLC is expressed in terms of mg of liquid/cu m of gas at 0° C. and 101.325 kPa.
Case Study 1
Table 1 shows the composition of a rich gas in the first two columns.
This rich gas contains 3.1 mole % C7+ with a molecular weight of 132 and a specific gravity of 0.774. The experimentally measured dewpoint pressure is 4,075.6 psia at 180.4° F.10 This case study will use the Peng-Robinson equation of state;11 the Soave-Redlich-Kwong equation of state gives similar results.
We used four different methods to characterize the C7+ components:
• Method A. This method treats C7+ as a single cut. Based on its molecular weight and specific gravity, we predicted its normal boiling point (317.2° F.), critical temperature (Tc = 645.2° F.), critical pressure (Pc = 371.6 psia), and acentric factor (ω = 0.425574) using correlations similar to the ones by Riazi and Daubert.12
The predicted dewpoint pressure is 3,433 psia-far away from the measured value of 4,076 psia.
Fig. 1 shows the predicted phase envelope for this method. This envelope does not pass through the measured dewpoint.
• Method B. This method breaks the C7+ into 11 single carbon numbers (SCNs) ranging from SCN 7 to SCN 17+ using the exponential decay procedure presented by Katz and applied by others.9 13 We used generalized SCN properties as reported by Whitson.14 Table 1 shows the resulting distributions.
The predicted dewpoint pressure is 4,030 psia, which is relatively accurate. Fig. 1 shows that the corresponding phase envelope almost passes through the measured dewpoint.
• Method C. This method lumps the 11 SCN components of Method B into four cuts. Table 1 also shows these cuts and compositions. The predicted dewpoint pressure is 3,885 psia. Fig. 1 shows the corresponding phase envelope.
• Method D. This method is similar to Method B, except that we used 12 normal paraffins (alkanes) instead of SCN components to represent the C6+ components. The predicted dewpoint pressure is 3,730 psia and Fig. 1 also shows the corresponding phase envelope.
The advantage of this method is that n-alkane components are readily available in many commercial software packages whereas the SCNs may not be.
Fig. 1 shows that none of the methods (except method B which comes close) matches the experimentally measured dewpoint. The next section shows how the prediction of each method can be improved to match the experimentally measured dewpoint.
Adjusting molecular weight
For Method A, by changing the molecular weight of C7+ to 152.5, we can match perfectly the measured dewpoint (Fig. 2). Based on a molecular weight of 152.5 and a specific gravity of 0.774, the new values for the estimated properties will be
• NBP = 381° F.
• Pc = 308.4 psia.
• Tc = 706.9° F.
• ω = 0.468456.
An alternative option is to adjust only Tc to 703.5° F. Again, the tuned Tc makes the phase envelope pass through the measured dewpoint (Fig. 2). The Tc adjustment is preferred because less work is involved.
Tuning interaction parameter
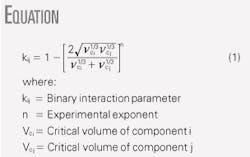
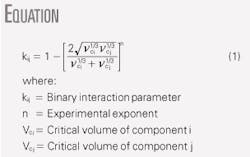
For Methods B and C, one can adjust the binary interaction parameter, kij. The accompanying box shows a common correlation to estimate the binary interaction parameter.
In this equation, Vci and Vcj represent the critical volumes of components i and j, respectively. The default value of exponent n is normally set to 1.2, but it can be used as a tuning parameter to match the experimentally measured dewpoint.
For our case study, we obtained n = 1.3011 for Method B and n = 1.6573 for Method C. Fig. 2 shows the resulting phase envelopes for these two methods.
Method D mole wt tuning
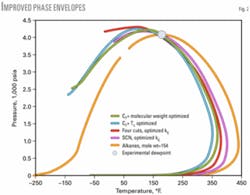
The distribution (i.e., the mole %) of the alkane part of C6+ depends on the assumed value of the C6+ molecular weight. Fig. 2 shows that, by changing the molecular weight of C7+ to 154, we can match perfectly the measured dewpoint.
Fig. 2 shows that all of the generated (tuned) phase envelopes pass through the measured dewpoint; but they have different cricondentherm points. Are all of these phase envelopes correct?
We performed Case Study 2 to answer this question.
Case Study 2
Table 2 shows the composition of a lean natural gas that we used in Case Study 2. This lean gas contains only 0.067 mole % C6+. Even though the amount of C6+ is small, it has a large impact on the condensate.
Table 2 (first two columns) shows the detailed laboratory analyses15 of C6+. We calculated the C6+ components to have a molecular weight of 94.12, a specific gravity of 0.738, and an NBP of 195° F. using the Soave-Redlich-Kwong equation of state.16 For this case, all other calculations were performed with the Soave-Redlich-Kwong equation of state.
We plotted the experimentally measured PHLC at 594.7 psia as a function of temperature in Fig. 1; extrapolation yielded a dewpoint temperature of 52.1° F. We used the methods from Case Study 1 to characterize the C6+ by matching the experimentally determined dewpoint.
Table 2 presents the tuned molecular weight and distribution of components for each method. Figs. 3 and 4 show the predicted PHLC as a function of temperature and the phase envelope, respectively. The cricondentherm for the lumped C6+ method was below 15° F.; therefore, we predicted zero values for PHLC.
Fig. 4 shows that three of the characterization methods generated practically the same phase envelope. But do all of these characterization methods present the true phase behavior of this lean gas? The answer is yes and no.
Fig. 3 indicates that, for temperatures close to the dewpoint, all three methods predict PHLCs close to the experimental values; however, at lower temperatures (about 20° F.) the deviation from experimental values increases. Only the normal alkane distribution method gives accurate values even at lower temperatures.
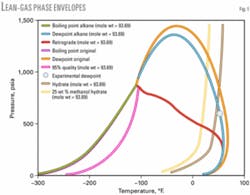
For this lean gas, Fig. 5 shows the complete phase envelope containing the hydrate curves, with and without inhibitor, a 95% quality line, and the retrograde curve for which C6+ is distributed based on n-alkanes.
Acknowledgment
The authors appreciate the support provided by John M. Campbell & Co. and Chemical Engineering Consultants Inc. We also acknowledge that the calculations were performed with WinProp (Computer Modeling Group), EzThermo (Moshfeghian & Maddox), and Gcap (John M. Campbell & Co.). ✦
References
1. Maddox, R.N., and Lilly, L.L., “Gas Conditioning and Processing, Volume 3: Advanced Techniques and Applications,” Norman, Okla.: John M. Campbell & Co., 1994.
2. Nasrifar, K., Bolland, O., and Moshfeghian, M., “Predicting Natural Gas Dew Points from 15 Equations of State,” Journal of Energy and Fuel, Vol. 19 (2005), pp. 561-72.
3. Danesh, A., “PVT and Phase behavior of Petroleum Reservoir Fluid,” 2nd Ed., Amsterdam: Elsevier, 2001.
4. Riazi, M.R., “Characterization and Properties of Petroleum Fractions,” Philadelphia: ASTM International, February 2005.
5. Shariati, A., Peters, C.J., and Moshfeghian, M., “A Systematic Approach to Characterize Gas Condensates and Light Petroleum Fractions,” J. of Fluid Phase Equilibria, Vol. 154 (1999), pp. 165-79.
6. Shariati, A., Peters, C.J., and Moshfeghian, M., “Further Evaluation of the Shariati-Peters-Moshfeghian C7+ Characterization Method,” J. of Fluid Phase Equilibria, Vol. 179 (2001), pp. 23-41.
7. Shariati, A., Moshfeghian, M., and Maddox, R.N., “The Effect of Correct C6+ Characterization on the Computer Simulation of Two-Phase Flow Pipelines,” International J. of Modeling and Simulation, Vol. 19 (1999), No. 4, pp. 352-56.
8. Starling, K.E., “Peng-Robinson Equation of State Natural Gas Dew Points,” presented at the American Gas Association Operations Conference, Orlando, Apr. 27-30, 2003.
9. Katz, D., “An Overview of Phase Behavior of Oil and Gas Production,” J. Petrol. Technol., June 1983, pp. 1205-14.
10. Firoozabadi, A., Hekim, Y., and Katz, D.L., “Reservoir depletion calculation for gas condensates,” Can. J. Chem. Eng., Vol. 56 (1978), pp. 610-15.
11. Robinson, D.B., and Peng, D.Y., “The characterization of the heptanes and heavier factions for the GPA Peng-Robinson programs,” Tulsa: Gas Processing Association, Research Report PR-28, 1978.
12. Riazi, M.R., and Daubert, T.E., “Simplify property predictions,” Hydrocarbon Processing, March 1980, p. 115-16.
13. Moshfeghian, M., Maddox, R.N., and Johannes, A.H., “A Consistent C6+ Distribution for Generation of Natural Gas Phase Envelope,” presented at the 16th International Congress of Chemical and Process Engineering, Prague, Aug. 22-26, 2004.
14. Whitsen, C.H., “Characterizing hydrocarbon plus fractions,” SPE J., Aug. 1983, pp. 683-93.
15. Derks, P.A.H., van der Meulen-Kuijk, L., and Smit, A.L.C., “Detailed Analysis of Natural Gas for an Improved Prediction of Condensation Behavior,” 72nd Annual Convention, Gas Processors Association, Tulsa, 1993.
16. Soave, G., “Equilibrium Constants from a Modified Redlich-Kwong Equation of State,” Chemical Engineering Science, Vol. 27 (1972), p. 1197.
The authors
Mahmood Moshfeghian ([email protected]) is a consultant and instructor at John M. Campbell & Co., Norman, Okla. He is on leave as a professor of chemical engineering at Shiraz University, Iran, where he served as department head and associate dean of research in the college of engineering. Moshfeghian was previously a professor of chemical engineering at the University of Qatar and a senior research scientist at the Kuwait Institute for Scientific Research. He holds BS, MS, and PhD degrees in chemical engineering from Oklahoma State University.
Larry L. Lilly (larry@ jmcampbell.com) is vice-president of John M. Campbell & Co. He has more than 30 years’ experience with engineering and consulting companies including design, commissioning, and startup. Lilly holds BS, MS, and PhD degrees in chemical engineering from Oklahoma State University. He is a member of AIChE, GPSA, GPA Europe and numerous local technical societies.
Robert N. Maddox (rmaddox @okstate.edu) is the Sheerar Chair emeritus professor of chemical engineering at Oklahoma State University. He served as head of the chemical engineering department from 1958 to 1978. He is a fellow of AIChE and a member of GPA.
Khashayar Nasrifar (khashayar. [email protected]) is currently a research associate at the Norwegian University of Science and Technology, Trondheim, Norway. He was formerly an assistant professor at University of Tehran, Iran. Nasrifar holds BS, MS, and PhD degrees in chemical engineering from Shiraz University, Iran.