Improved field test measures polyacrylamide fluid
Practical Drilling Technology
Scientists have developed a safer, faster, more cost-efficient method for analyzing polyacrylamides in drilling fluids, suitable for field use.
As a result of increasing global emphasis on minimizing the environmental impact of drilling, many operators utilize water-based drilling fluids. In order for these fluids to obtain a performance similar to oil- and synthetic-based fluids, the formulations include cutting-edge synthetic polymers that, when added to aqueous drilling fluids, can impart useful properties even at low concentrations.1-4
The equipment used for PA analysis appears in a top view of the new apparatus (Fig. 1a, above left); the apparatus setup on HTHP (Fig. 1b, above right); and the original glassware setup (Fig. 1c, below).
null
null
Without careful control of these small concentrations, over treatment can result in adverse side effects such as increased viscosity and more importantly increased operator costs. Knowing the exact concentrations of the polymers in the active system is vital for achieving cost-effective performance. However, polymer concentrations are often estimated based on product concentrations and cuttings volume–obviously not allowing the operator the best value.
Polyacrylamide is one such widely used synthetic polymer. The polyacrylamides and related polymers have found several applications in water-based fluids including control of cuttings erosion, flocculation, rheology, and wellbore stability.5-8 Use of synthetic polymer systems is rapidly increasing, but the supporting technologies such as fluid testing had not moved forward at a similar rate.
Recent development of an apparatus and improved technique for accurate analysis of polyacrylamide (PA), partially hydrolyzed PA (PHPA), and related materials containing amide functionalities allows operators and drilling fluid specialists to determine exact concentrations in the active system.
The current technique used in the field for PA/PHPA testing centers on a caustic digestion of the whole drilling fluid, followed by collection of ammonia in a boric acid solution, and back titration with sulfuric acid to determine the PA content.9
In order to undertake this analysis, the fluids specialist uses a set-up consisting of glass flasks, condensers, and rubber hoses. While the chemistry behind this test is safe, there are many problems with the current test kit, including: (a) loose or cracked hoses that release ammonia in the vicinity of workers; (b) fragile glassware which is easily broken on a rig site; (c) the time required to complete the test; and (d) inconsistent results because of super-heating, or "bumping", of the basic digestion solution. As a result, rig hands and field mud specialists are hesitant to use this analysis method.
The initial aim of the research reported here was the design of a field-friendly apparatus that solved each of these significant problems, yet required few changes to existing techniques and minimal extra equipment on the rig site. Figs. 1a and 1b outline the final design. The new test overcomes many of the inherent PA-test problems by combining all the reaction vessels currently used (Fig. 1c) into a single piece that is made of steel/PVC and connected by gas-tight stem valves. The new procedure provides both improved consistency in results and greater accuracy.
Testing procedure, methodology
The testing procedure incorporates the materials listed in Table 1.
An HTHP jacket is preheated to 285º F. (141º C.). A stem valve is inserted and closed on a dry HTHP cell. This cell is inverted and placed in a stand. Whole mud (5 ml) is then added, along with DI water (55 ml), and NaOH (5 ml, 5N). The lid is placed on the cell and locked into place with the setscrews.
The apparatus stem-valve is then screwed into place on the HTHP cell. Boric acid (30 ml, 3% aqueous solution) and 6-10 drops of bromocresol green-methyl red indicator are both added to the white bowl of the titration apparatus. The titration apparatus is then locked onto the stem-valve with the cotter pin. The whole apparatus is placed into the preheated jacket and a 30-min timer is started upon the appearance of the first bubble.
After 30 min, the cotter pin is removed, the bowl placed aside on its base, and the HTHP cell is removed from the jacket. Titration of the boric acid solution can then be carried using H2SO4 (N/50). The solution should turn from a dark green back to a pink color. The pounds per barrel (ppb) of active material can then be calculated from a calibration curve previously determined with standard solutions. Washing with soapy water easily cleans the whole apparatus when it is cool enough to handle.
Results
Polyacrylamide samples were calibrated using 10 ml samples of PA solutions (Fig. 2). The reproducibility of these tests is very high. Six analyses of the same 4-ppb solution resulted in a small variance of ±1.0 ml acid (which represents a total range of only 0.25 ppb).
While the PA titration apparatus was known to be successful for standard solutions, it had to be tested against actual drilling fluid filtrates and whole mud samples in order to confirm its usefulness as a fluid test. A sample set of results is presented below. The titration apparatus and methodology have been double blind tested by several formulators and engineers, resulting in similar, accurate results in each case.
null
Filtrate, whole mud samples
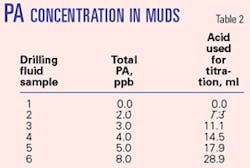
A typical PA-based fluid is used, where the only variance in the formulation is the total amount of polyacrylamide. Table 2 notes the polyacrylamide concentration in muds—the amount of acid required to titrate PA during analysis.
The total polyacrylamide content of these mud samples progresses linearly, as does the amount of acid required to titrate, indicating that we get consistent digestion of the polyacrylamide, even with increased loading. Typical PA concentrations fall inside the 0-8 ppb range tested here, but higher concentrations can be analyzed with the same accuracy. A safer, faster, more cost-efficient method for analyzing polyacrylamides has been outlined. The apparatus is more robust than current test kits and is suitable for field use. In addition, the analysis can be successfully utilized with whole muds, filtrates, or standard polymer solutions.
The test has produced results that are drastically more reproducible (±0.25 ppb on a given sample) than the current test employed, while obtaining the same results accuracy. This particular test is not limited to polyacrylamides. Its compatibility with other drilling fluid additives is currently under study.
References
1. Alford, S., "North Sea Field Application of an Environmentally Responsible Water-Base Shale Stabilizing System," SPE Conference, Amsterdam, Mar. 11-14, 1991, Paper 21936.
2. Yabin, N., Daming, Z., Honzhang, X., Jian, L., "Research and Application of Metal Complex-Amphoteric Polymer Drilling Fluid," SPE Conference, Beijing, Nov. 2-6, 1998, Paper 50932.
3. Mas, M., Tapin, T., Maqruez, R., Gabay, R., Negrin, Z., Diaz, C., Bejarano, L., "A New High-Temperature Oil-Based Drilling Fluid," SPE Conference, Caracas, Apr. 21-23, 1999, Paper 53941.
4. Thaemlitz, C.J., Patel, A.D., Coffin, G., Conn, L., "New Environmentally Safe High-Temperature Water-Based Drilling-Fluid System," L. Drill. & Completion, 1999, pp. 185-189.
5. Hill, D.G., "Clay Stabilization— Criteria for Best Performance," SPE Conference, Dallas, Mar. 24-25, 1982, Paper 10656.
6. Denny, J.P., Shannon, J.L., "New Way to Inhibit Troublesome Shales," World Oil, 1968, 117, pp. 111-112.
7. Loklinham, G., "The Drilling Fluid Inhibition Properties Effect on Hole Quality—A Well Survey," SPE Conference, Dallas, Feb. 26-28, 2002, Paper 74544.
8. Grebe, L., "Development of Deepwater Water-Based Drilling Fluid," Halliburton-Baroid Technology E & D Labs, 2002.
9. Baroid Drilling Fluids, I. Baroid Fluids Handbook, Houston, 1999, pp. 5.62-5.64.
The author
Stephen Bell (stephen.bell@ halliburton.com) is senior scientist at Halliburton Baroid, Houston. He has also served as a post-doctoral researcher at Texas A&M University, 2001-03. Bell holds a PhD in chemistry from the University of Pittsburgh (PA) and a BSc in Chemistry from the University of Sussex (Brighton, England). He is a member of SPE, the American Chemical Society (ACS), and the American Association for the Advancement of Science (AAAS).