Underperformance of ULSD units may create supply problems in US
S.J. McGovern
C.K. Lee
PetroTech Consultants
Mantua, NJ
J.A. Zagorski
Middough Consulting Inc.
Philadelphia
Although distillate desulfurization chemistry is fairly well understood, there are still inconsistencies in the operation of small-scale catalyst testing units and in the scale-up and design of large, high-conversion trickle bed reactors that produce ultralow-sulfur diesel (USLD). As a result, some units will perform better than expected, while others will not meet their design requirements, causing a potential shortfall in the production of ULSD.
This article discusses the reasons for unit underperformance and outlines possible solutions.
To meet new sulfur regulations, refiners have made multimillion-dollar investments in a short time. The industry received advice from process licensors, engineering and constructing contractors, and catalyst vendors. Although all of these sources provided valuable information, there seems to be no full consensus on some of the important design considerations.
Larger companies have corporate research and engineering departments that can help sort through this maze of information, but even they had to make decisions based on incomplete or conflicting information.
Specifications
Fuel-sulfur levels are being reduced throughout the world. Gasoline and diesel fuel are currently limited to 50-ppm sulfur in Europe and will be reduced to 10-ppm sulfur in 2009. Several countries have encouraged the early introduction of 10-ppm sulfur fuels through tax credits. Heating oil and off-road diesel are currently limited to 2,000-ppm sulfur in Europe; off-road diesel will be reduced to 10-ppm sulfur in 2009.
The US is also requiring lower fuel-sulfur levels. The phase-in of lower-sulfur gasoline began in 2004 and most US gasoline will have an average sulfur content of 30 ppm by 2006. On-road diesel must be at 15-ppm sulfur by mid-2006 and the various off-road diesel sulfur contents will be lowered to 15 ppm between 2007 and 2012.
These new fuel-sulfur regulations will increase the importance of hydrotreating. The ultralow-sulfur requirements will make it almost impossible for a refiner to produce these products unless the desulfurization units are operating as designed.
Less demand for higher-sulfur products greatly reduces the current flexibility that refiners have in producing other higher-sulfur products, especially when a desulfurization unit must be shut down for catalyst replacement or mechanical repairs. In the near future, unscheduled desulfurization unit shutdowns might result in entire refinery outages. In addition to current refinery projects, the US Environmental Protection Agency estimates that in the US, 60 new hydrotreaters and 40 revamps will be required for the off-road diesel specs that begin in 2007. If the sulfur content of home heating oil or jet fuel is also lowered, then even more new hydrodesulfurization (HDS) units will be required.
Although Europe is considering lowering heating-oil-sulfur content throughout the European Union, the US government does not regulate the sulfur content of heating oil. Instead, individual states set the level. Several states have proposed reductions, but none has yet been legislated. Currently, some low-sulfur diesel (LSD) is sold as premium heating oil.
Jet fuel may contain up to 3,000-ppm sulfur. The average sulfur content of most jet fuel, however, is slightly more than 500 ppm; some current production is significantly lower in sulfur. Although there is no pending legislation to lower the sulfur content of jet fuel, a reduction to 500 ppm would affect only a small portion of the product volume.
In the US, any kerosine that will be blended into ULSD must also be certified to have been produced and transported as ULSD with less than 15-ppm sulfur. EPA has recently proposed a regulation reducing the sulfur content of the fuels used by stationary turbines to 500 ppm.
Design issues
The design bases for new and revamped ULSD units under construction have mostly been developed from data obtained in small-scale catalyst testing units. Although all commercial HDS units operate with the liquid flowing down through the catalyst bed, many of the catalyst testing units operate with the liquid flowing upwards.1 This operating mode is often preferred because it gives more reproducible results and is less dependent on the reactor-loading technique. Reproducibility, however, does not guarantee accuracy. Fluid dynamics and mass-transport rates in a laboratory-scale, upflow reactor are much different than those of a commercial downflow, trickle-bed reactor. The vapor-liquid mass transport rates can be higher and the liquid phase residence times can be different.
Operating laboratory testing units in downflow mode is preferred; however, obtaining reproducible results that predict commercial performance largely depends on the quality of the technique used to load catalyst into the reactor. Each laboratory has its own technique, developed via experimentation.2 There is no guarantee that any technique will accurately predict commercial unit performance for ULSD conditions.
During the past 40 years, numerous articles and books have been published on the scale-up, design, and operation of trickle-bed reactors. Most of these articles are based on data developed in small diameter, lab-scale equipment at conditions far different than commercial HDS units. Less than 5% of the mass transport data have been developed in units that are more than a few inches in diameter.
There are a few articles that have presented commercial HDS data, generally showing large improvements in commercial reactor efficiency by installing improved, initial gas-liquid distribution systems. Most of these articles, however, do not show a direct comparison of commercial and lab-scale performance.
Hydrotreating technology has been used in refineries for more than 50 years, but it is still not completely understood, especially in the area of reactor design and scale-up.
Before introduction of LSD in the early 1990s, basic hydrotreating units for treating naphtha, kerosine, and distillate were relatively simple, low-pressure, inexpensive units. They were either gas phase (for naphtha and kerosine) or small, trickle-bed reactors with relatively low treat-gas rates.
In the trickle-bed reactors, the oil flows down through the catalyst bed as a liquid phase, while the hydrogen-rich treating gas flows cocurrently in the vapor phase. Some of the oil, along with the lighter sulfur compounds, vaporizes into the gas phase; but most of the sulfur-containing molecules, especially the hindered dibenzothiophenes and polynuclear aromatics, remain in the liquid phase.
Lessons learned
When 500-ppm sulfur LSD was required in 1993, some new and revamped HDS units did not operate as expected,3 which caused an initial shortage of LSD. Before the LSD regulations, most diesel HDS units operated at less than 80% desulfurization and a significant fraction of the US diesel production required little or no desulfurization.
The LSD regulations required many units to operate at more than 90% desulfurization. Industry quickly learned that small amounts of liquid bypassing, caused by poorly designed or installed initial distributor trays, made it impossible to produce a 500-ppm sulfur product, even with the most active catalyst. Surprisingly, reducing the feed rate in an attempt to make some low-sulfur product in these units sometimes worsened the problem.
Some of the early HDS units used rudimentary spray devices in the reactor’s inlet nozzle to distribute the liquid to the bed’s surface. Although a few of these are still operating, most have either been replaced or supplemented by one or more full-diameter distribution trays close to the bed surface.
Several articles, published since the introduction of LSD, show that installation of improved liquid distribution systems greatly improved unit performance (OGJ, Sept. 10, 2001, p. 68).4 5
The patent literature contains many vapor-liquid distributor designs for commercial scale, trickle-bed reactors. Other proprietary designs are also available. These trays consist of many small (to fit through the reactor’s inlet nozzle) flat plates that are assembled in the reactor to form a fluid-tight seal against the reactor wall.
The individual tray sections are bolted or pinned together and attached to support beams. The methods of tray support and sealing vary with tray designer and manufacturer, but a liquid tight seal is crucial. The tray sections each contain many individual liquid and gas distribution devices. Many of these designs claim uniform liquid distribution during laboratory testing. Actual commercial performance, however, is impossible to confirm.
Table 1 compares the typical density of distribution points in a lab-scale reactor and a “state-of-the-art” distributor tray. Even the current designs do not perform as well as a typical pilot unit. Some designs provide a spray from the bottom of each device to improve coverage. There are other aspects of tray design, however, that can cause nonuniform coverage of the catalyst bed:
• Out-of-level trays caused by manufacturing or installation tolerances (typically 1⁄2 in. for large-diameter reactors).
• Liquid leaks between the tray sections due to poor installation.
• Poor liquid coverage near the reactor wall.
• Liquid gradient across the tray caused by flow restrictions.
• Interference of the tray support beams.
The lab-scale testing results from Swain and Zonnevylle4 and Nocca5 for improved tray designs show large improvements in liquid distribution. The distributions, however, are still not completely uniform, even under ideal laboratory conditions. Regardless of the tray design, it is impossible to achieve uniform initial liquid distribution in a commercial hydrotreating reactor.
Inerts and catalyst at the bed’s top are often used to mitigate pressure drop build-up and to attempt to distribute the liquid flow better; however, the measured liquid spreading rate in two-phase, cocurrent flow is low. It is typically less than 0.1 in./ft for 3-mm particles.6
The spreading rate increases for larger particles, but particles with holes tend to have spreading rates like smaller particles.
With pending ULSD requirements, some hydrotreaters will be operating at desulfurization levels exceeding 99.95%; many hydrotreaters currently operate at less than 90% HDS. Designing and operating a fixed-bed hydrotreater at 99.95% HDS will be difficult.
Small amounts of bypassing in the reactor, leaking in the feed-effluent exchangers, or inadequate H2S stripping can lead to off-spec product. Operating laboratory units at these desulfurization levels has even proven to be difficult.
Additional difficulties
Many studies have confirmed that the key to producing ULSD is desulfurizing the hindered dibenzothiophenes (HDBT). Because of their structure, they are more easily desulfurized if they are first hydrogenated. This is most easily accomplished at higher hydrogen partial pressures and higher treat-gas ratios.
Hydrogen consumption in a ULSD unit will be higher than it is in an LSD unit. Refiners that do not have the pressure in their existing units effectively to desulfurize the HDBTs must either build new units or adjust the feed to the diesel unit to move the HDBTs into off-road diesel.
Most of the published information concerning ULSD technology is based on laboratory data. Few articles have shown actual commercial data. ULSD is currently produced in several European countries and in several US refineries.
The European refineries generally process lighter, lower-sulfur crudes than the US refineries. They also have less FCC and coking capacity; therefore, their distillates are also lower in aromatics and nitrogen and are easier to desulfurize with less HDBTs and lower hydrogen consumption. The few US refineries that are currently producing ULSD are tailoring the feeds to produce ULSD from existing equipment.
At typical ULSD conditions, the solubility of hydrogen in the oil phase is less than 50 scf/bbl and hydrogen consumption can be more than 300 scf/bbl. Because the HDBTs are in the liquid phase in the reactor, the mass-transfer rate of hydrogen into and through the liquid phase can limit HDBT desulfurization.
All current trickle bed mass-transfer correlations have been developed for conditions that do not represent those actually encountered in commercial units.7 Essentially all ULSD units operate in the “low-interaction” flow regime, whereas the bulk of the mass-transport data has been developed in the “high-interaction” regime.
Existing correlations show that the transport of hydrogen through the liquid film on the catalyst will not significantly reduce the overall desulfurization rate.8 These correlations, however, can overpredict the mass-transfer rate by more than a factor of 10. Lower mass-transport rates can reduce significantly the effective desulfurization rate of a commercial unit. This is especially true for high-activity catalysts.9
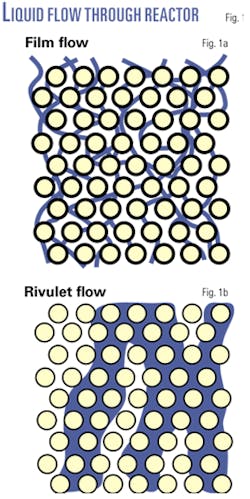
Only a few researchers have investigated the true quality of liquid flow in trickle-bed reactors at commercially relevant flow rates. Those that have conclude that the liquid does not flow through the bed as a uniform film on the catalyst surface.10-12
Even with initial distribution uniform to the scale of the individual particles, much of the liquid flow quickly coagulates into rivulets of liquid that are several particles thick, excluding much of the treat gas from these regions (Fig. 1).
This rivulet flow is quickly established at a depth of 5-10 particles. The rivulets can change location, but they are highly stable and their number and size are reproducible. They are independent of the wettablity of the particles and only weakly dependent on the liquid properties.
The liquid velocity in these rivulets is higher than in the rest of the bed. Rivulet flow occurs even at the mass flux of 3,000 lb/hr-sq ft often recommended for trickle-bed reactors.
The rivulets cause three effects that will decrease the desulfurization performance of a trickle-bed reactor:
• Reduced average liquid residence time in the bed (an increase in effective space velocity) especially compared with upflow lab reactors (Fig. 2).
• Longer diffusion path length for the hydrogen (and H2S) to traverse, which results in a lower hydrogen and higher HS concentration at the catalyst surface.
• Reduced local treat gas rate within the rivulets. Pressure drops within the catalyst bed cause the gas flow to migrate to regions of low liquid flow; therefore, there is a distribution of treat-gas ratios and space velocities (Fig. 3).
The regions of highest space velocity have the lowest treat-gas rates. These effects cause a significant reduction in local reaction rates within the rivulets. The net effect is equivalent to feed bypassing.
The sizes of the rivulets are proportional to the particle sizes through which they are flowing. Small inerts that are used in lab-scale units can reduce the size of the rivulets to smaller than the catalyst particle. This increases the mass-transport rate and improves catalyst performance.
Using large inerts on top of a commercial reactor will reduce macro-maldistribution (i.e., they improve the initial liquid distribution), but they can cause well-distributed liquid to clump into larger rivulets before entering the catalyst bed. Accumulated scale at the bed’s top can also cause liquid maldistribution. These effects must be balanced when developing a reactor-loading scheme.
Commercial unit
The most common symptom of maldistribution in a commercial HDS unit is sub-par performance. The unit will require a higher start-of-run temperature and experience greater catalyst-deactivation rates. Reactor maldistribution often causes poor performance of improved HDS catalysts.
Older units may have lower performance due to maldistribution; however, the refinery may have downrated the unit so the low performance is now expected. The effects of maldistribution might only become apparent as the unit is pushed to higher desulfurization levels, more cracked stock is processed, or better catalysts are used.
Large performance improvements have been documented for new distributor tray systems. These improvements mean that maldistribution is reduced but not necessarily eliminated.
Finding additional evidence to support the diagnosis of liquid maldistribution in a commercial unit can be difficult. One can diagnose severe, large-scale maldistribution by identifying temperature variations in the bed, using catalyst-sampling programs, or using radioactive tracers or gamma scans. These techniques cannot detect less-severe or small-scale maldistribution.
Computational fluid dynamics calculations also have limited value and only identify large-scale maldistribution caused by the distributor tray design. The computational scale required to model smaller-scale maldistribution within the bed is beyond current technology. The fine-scale interactions of the vapor, liquid, and solid phases cause most of the unusual behavior of trickle-bed reactors. To model this behavior properly, the elements of the computational grid must be smaller than the catalyst particles. This leads to computational problems.
Most current models use calculation grids containing about 100,000 nodes. Dividing the length scale of a 1.3-mm catalyst particle into three nodes would only allow a 1⁄2-in. diameter bed for a 4:1 length:diameter reactor to be modeled with 100,000 nodes. A 1-ft diameter bed would require more than 1 billion nodes.
The liquid-vapor interactions are even more difficult to model. Most attempts to model flow in a packed bed treat the two phases as a single pseudophase with averaged properties. This eliminates the model’s ability to demonstrate small-scale maldistribution caused by phase segregation during flow through the bed.
The most reliable method to identify poor commercial HDS reactor performance is to simulate the commercial unit operation in a small-scale laboratory unit. If the same feed and catalyst are used with the same start-up and operating conditions, then the primary difference between the two operations is the hydrodynamics or mass-transport characteristics of the two units.
If these tests are performed correctly, they can identify differences as small as 10° F. (80% effective catalyst utilization). The key to the success of these tests is good lab unit operation with very small inerts.
Solutions
With LSD, the industry learned that “macroscale” maldistribution, caused by poorly designed or maintained distributor trays, can cause an HDS unit to miss its production targets. With ULSD, units must also be able to avoid “micro-scale” as well as macroscale maldistribution.
Micro maldistribution is a function of the bed type and flow conditions; the initial vapor-liquid distribution system does not control micro maldistribution. A well-designed initial distribution system is required for ULSD but does not guarantee good ULSD performance. Other aspects of reactor design and catalyst loading have an influence.
Microscale maldistribution (rivulet flow) is present in all units and macro-maldistribution (initial distribution) can only be reduced but not eliminated; therefore, a ULSD unit’s design must recognize these facts. This is usually done in one of two ways: additional catalyst volume, treat gas rate, or reactor pressure is added to the design as a “safety factor” or a multibed reactor system is used.
The amount of additional design margin required to compensate for unavoidable maldistribution cannot be accurately calculated. The current safety margins for LSD units have been developed through experience, by comparing actual commercial unit performance with predicted performance based on lab scale testing.
Commercial units usually do underperform the lab units, which has resulted in the design margins. The design margins account for all of the nonidealities of a commercial unit, including maldistribution and mass-transport limitations that can result in localized hydrogen starvation within the bed.
There is little experience, however, in scaling up ULSD units; current design factors are based on lower HDS levels and lower hydrogen consumption. These might not be sufficient for the higher-severity ULSD operations.
The negative effects of maldistribution and mass-transport limitations become larger as the extent of desulfurization or hydrogen consumption increase and as more active catalysts are used.
Most commercial ULSD trials in the US have tailored the feed to the unit to minimize the HDBTs in the feed. Refiners do this by reducing the amount of light cycle oil in the feed or lowering the feed end point, resulting in lower hydrogen demands for the reaction system. Eliminating the HDBTs from the feed will mask some of the problems that could be present with full-range ULSD production.
It is difficult to develop ULSD design margins with existing units. Most existing units cannot produce ULSD without large deviations from normal operations. Commercial tests must be conducted at several different conditions with different feeds; complete analyses are needed, including comparisons with appropriate lab scale testing to develop reliable design margins.
The multibed reactor system is an alternative to design margins. It is effective for ULSD production because it addresses all the problems that micro and macro maldistribution can cause and its effects are predictable.
Units that use multiple beds usually perform better than lab-scale results would predict, which eliminates the need for design margins.9 Rost and Lee demonstrated that ULSD can be produced in a multibed reactor (OGJ, June 2, 2003, p. 52).13
Basic reaction engineering theory shows that a system of three poorly operating beds in series with good interbed mixing performs almost as well as an ideal plug flow reactor. The total catalyst fill is divided among the three beds. The extent of reaction is limited in each bed; therefore, the amount of hydrogen that must be transferred to the liquid in each bed is also lower.
Temperature gradients and sulfur concentration gradients are also lower. After each catalyst bed, the liquid and vapor must be well mixed before being redistributed into the next bed. The mixing and redistribution ensures a uniform distribution of sulfur and temperature across the next bed and allows the liquid phase to be resaturated with hydrogen.
Good liquid mixing between beds is critical for the multiple-bed strategy. There are many interbed mixing devices available. Devices that swirl all of the liquid provide better mixing than liquid impingement devices.14 Also, there must be sufficient liquid residence time in the swirl zone to provide adequate mixing.
A well-designed interbed mixing and redistribution system is no more than 5 ft high. Adding two mixing and redistribution zones to a 40,000-b/d unit would increase the reactor size by less than 20%. This is significantly less than typical design margins.
As previously mentioned, the bed-topping materials and the particles that they collect will cause maldistribution. Some of the activity loss that a desulfurization unit experiences is not due to catalyst coking but to a progressive increase in maldistribution caused by the fines accumulating at the bed’s top.
Although reticulated topping materials are proving to have better collection efficiencies, fines deposition at a bed’s top will always cause additional maldistribution and a performance loss. Using a multibed reactor prevents this maldistribution from affecting the performance of the whole reactor. The lower beds are free from the maldistribution caused by accumulating fines in the top bed.
The one-time cost of designing and installing a multibed reactor is quickly recovered via longer cycles and lower operating costs. Designs with a larger catalyst volume, higher treat-gas rate, or higher pressure have higher operating costs. A multibed reactor can have a lower initial cost if it can eliminate the large safety margin included in the single-bed design. Multibed reactors often perform better than even the best lab-scale reactors. ✦
References
1. DeWind, M., et al., “Upflow versus Downflow Testing of Hydrotreating Cataslyst,”Applied Catalysis, Vol. 43, 1988, p. 239.
2. Al-Dahhan, M.H., and Dudukovic, M.P., “Catalyst Bed Dilution for Improving Catalyst Wetting in Laboratory Trickle-Bed Reactors,” AIChE Journal, Vol. 42, 1996, p. 2594.
3. Yeary, D.L., Wrisberg, J., and Moyse, B.M., “Revamp for Low Sulfur Diesel: A Case Study,” 1997 NPRA Annual Meeting, Mar. 16-18, 1997, San Antonio.
4. Swain, J., and Zonnevylle, M., “Are you really getting the most from your hydroprocessing reactors?,” European Refining Technology Conference, Nov. 15, 2000, Rome.
5. Nocca, J.L., et al., “Ultra-low sulfur diesel with the Prime-D technology package,” 2003 NPRA Annual Meeting, Mar. 23-25, 2003, San Antonio.
6. Anderson, D.H., and Sapre, A.V., “Trickle-Bed Reactor Flow Simulation,” AIChE Journal, Vol. 37, 1991, p. 377.
7. Larachi, F., et al., Industrial and Engineering Chemistry, Research 42, 2003, p. 222.
8. Jones, L., and Kokayeff, P., “Distillate Hydrotreating to ULSD, the Impact of Aromatics,” 2004 AIChE Spring National Meeting, Apr. 25-29, 2004, New Orleans.
9. Derr, W.R., et al., “The Role of Trickle Bed Reactor Design in Meeting Future Clean Fules Regulations,” World Refining, October 2002.
10. Hoek, P.J., et al., “Small Scale and Large Scale Liquid Maldistribution in Packed Columns,” Chem. Eng. Res. Des., Vol. 64, 1986, p. 431.
11. Christensen, G., et al., “Cocurrent Downflow of Air and Water in a Two-Dimensional Packed Column,” AIChE Journal, Vol. 32, 1986, p. 1677.
12. Wang, Y-F., et al., “Scale and Variance of Radial Liquid Maldistribution in Trickle Beds,” Chemical Engineering Science, Vol. 53 (1998), No. 6, p. 1153.
13. Lee, C.K., Pracht, O., Kossol, R., and Sangl, J., “Revamping hydrotreaters to produce ULSD: Bayernoil’s experience,” 2003 NPRA Clean Fuels Conference, Aug. 22-23, 2003, Houston.
14. Sarli, M.S., McGovern, S.J., Lewis, D.W., and Snyder, D.W., “Improved hydrocracker temperature control: Mobil quench zone technology,” 1993 NPRA Annual Meeting, Mar. 21-23, 1993, San Antonio.
The authors
Steve McGovern (sjmcgovern @hotmail.com) is a principal of PetroTech Consultants, Mantua, NJ. His areas of expertise include hydroprocessing, catalytic cracking, clean fuel technologies, and reactor design. Previously, he was a technology expert for Mobil Technology Co. where he worked for 27 years, primarily in process development and commercial operations support. McGovern holds a BS in chemical engineering from Drexel University, Philadelphia, and a PhD in chemical engineering from Princeton University, NJ. He is also a director of the Fuels and Petrochemicals Division of AIChE.
C.K. Lee is a principal of PetroTech Consultants, Mantua, NJ. His areas of expertise are hydroprocessing, catalytic reforming, and clean fuel technologies. Previously, he worked for Mobil Technology Co. for more than 20 years. Lee holds a BS in chemical engineering from Cheng Kung University, Taiwan, and a PhD in chemical engineering from the University of Houston.
John A. Zagorski (zagorsja @middough.com) is a senior technical manager at Middough Consulting Inc., Philadelphia. He has 28 years’ experience in petroleum refining in operations and as a consulting engineer. For 19 years, Zagorski worked for Mobil Oil Corp. and Coastal Eagle Point Oil Co. After leaving Coastal, he worked as a consultant for Western ROPE LLC, C&I Engineering, Jacobs Engineering Group Inc., and most recently Middough Consulting. Zagorski holds a BS in chemical engineering from Lehigh University, Bethlehem, Pa.