Permafrost Cement - Conclusion: Ceramicrete blends produce strong, low-permeability cements for arctic use
Researchers at Argonne National Laboratory in Illinois, have determined that blending ceramicrete with extendospheres and wollastonite or ash produces a superior cement for extremely cold climates. The cement is especially suitable for projects in permafrost because of its very low thermal conductivity.
Ceramicrete is a composition of magnesium oxide and monopotassium phosphate. Using fly ash as an extender, a low thermal conductivity of 0.27 w/mK. (watts/meter Kelvin) has been achieved. Addition of extendospheres further reduces this conductivity to 0.2 w/mK. The heat of formation is comparable to the current cement formulations. The set cement is free of connected pores and hence the water permeability is as low as 0.004 md.
Consistometer tests show that at least a 3 hr thickening (pumping) time is achievable and the set cement has a compressive strength of 1,276 psi, which is adequate for oil well cement. Using wollastonite as an extender, this strength can further be improved. Such properties allow this cement to be used in such applications as downhole drilling operations, in pipeline supports, and as an insulating material in cold regions.
Cement formulation, testing
A good permafrost cement should provide a pumping time of about 3 hr and will harden in less than 24 hr. We assessed a number of compositions in a chilled consistometer according to American Petroleum Institute Specification 10.4 To test the pumpability of this cement, the slurry was formed by adding the powder mix to the water in a Hobart tabletop mixer that was run at 30 rpm. The powder mixture was refrigerated at 25˚ F. prior to test. The mixing was done for 5 min and the resulting slurry was poured in the consistometer slurry cup (supplied by Chandler Engineering Co., Tulsa).
The consistometer was connected to a chiller (Thermo Neslab m75). The minimum steady temperature that could be reached was -1° C. (30° F.), which is below the freezing point of water, and hence, the tests were conducted around this temperature. The pressure was maintained at 1,500 psi, typical of a shallow well.
Once the consistency had reached 70 Bearden units or the test was run for 5 hr, the slurry was taken out and poured in the ASTM standard cylindrical molds. A small amount of water was poured on the surface of each sample and was kept in the refrigerator for curing.
Table 1 lists the ceramicrete compositions and results of each test. The first three tests are for a stoichiometric composition of MgO and KH2PO4, as dictated by the stoichiometry in the equation:
MgO + KH2PO4 + 5 H2O = MgKPO4∙6H2O
The first test showed that the amount of KH2PO4 was not enough to lower the freezing point of water sufficiently, and the slurry froze. When the content of the total binder and hence of KH2PO4 was increased, the freezing point of the slurry fell sufficiently to yield a pumping time longer than 5 hr. At the same time, its hardening also took much longer.
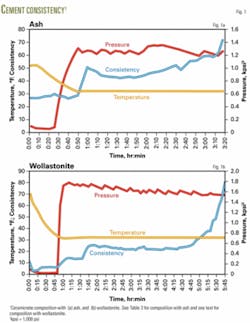
To reduce the pumping time and accelerate the setting, we increased the amount of MgO. More MgO provides more surface area for the acid-base reaction, and that accelerates setting of the slurry. We found that an equal amount of MgO and KH2PO4 gives a pumping time of 3 hr and 25 min and that the slurry hardens within hours in the permafrost environment. Fig. 1 gives the time vs. consistency graph for the last case in Table 1. Several conclusions may be drawn from these tests:
1. The minimum amount of binder needed for good permafrost cement is 50 wt %. When the binder content in the slurry is 40 wt %, the slurry did not harden in 24 hr.
2. Increased MgO content reduces the pumping time. As MgO content increased, the thickening as well as hardening time became shorter. Thus, adjusting the MgO content allows one to obtain desired pumping and setting times.
Properties of set cement
The slurry from the consistometer in the third test in Table 1 was cured for 72 hr at -4° C. (25° F.) and its properties were determined after thawing the specimens. The open porosity was determined by the water immersion method. The compressive strength and water permeability were measured with an Instron machine and the Chandler cement permeameter, respectively. Table 2 presents the results.
The density is nearly the same as that of the ceramicrete cured at room temperature (Table 2). The open porosity is also very low. The compressive strength is 8.8 MPa (1,276 psi), much lower than that of the sample cured at room temperature. The reduced compressive strength must be due to its curing in water, which leaves a significant amount of hydrated phases in the material.
This finding needs to be investigated. The actual strength, however, is adequate for downhole applications, where typically even 3.5 MPa suffices. As we shall see below, substituting calcium silicate for Class F ash can increase the strength.
The water permeability is extremely low, 0.004 md. The typical value of water permeability for commercial oil-well cement is 0.1 md.11 The low value is consistent with the low porosity of these cements.
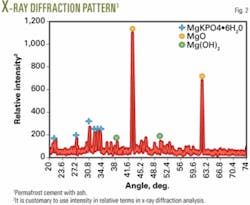
Fig. 2 gives the X-ray diffraction output of the cured cement. Note the cement has a considerable amount of unreacted MgO and, also because of water curing, some Mg(OH)2 has formed. The binding phase is MgKPO4·6H2O as expected. Thus, this cement is a composition of ceramicrete binder, MgO, and ash.
One of the effects of this excess MgO in the diffraction patterns is that it makes peaks of ash components such as silica very weak and hence they are not visible in the diffraction pattern. We expected the silica peak to be at -28° C.9, but the low concentration of silica compared to MgO obscured it in the pattern.
Effect of downhole gases
The problem with calcium silicate-based conventional cement in the downhole hydrocarbon environment, particularly in gas- hydrate regions, is that setting is hindered by calcium oxide that forms calcium carbonate. The ceramicrete-based cement, by contrast, contains MgO that is less susceptible to carbonation than CaO and, hence, should not be affected by the hydrocarbon environment.
To test this hypothesis, we used dry ice to produce carbon dioxide. Some dry ice was mixed with the slurry, and the test was run in the consistometer. The pressure in the slurry cup was built up at the end of the test, which indicated that ice had formed carbon dioxide.
The slurry cured in a refrigerator by being placing it in a container that was full of dry ice. In spite of the exposure to carbon dioxide, the slurry set well the next day. From this test one may conclude that setting of ceramicrete-based permafrost cement is not affected by downhole gases.
Incorporating extendospheres
Reducing the thermal conductivity of the cement further involved use of extendospheres composed of silica spheres separated from fly ash. They were provided by PQ Corp. and labeled as Q-CEL 6042. For this test, we used the slurry composition given in the last row in Table 3 and added different proportions of the extendospheres by weight.
Tests with 5 - 20 wt % extendospheres showed that the maximum amount of extendospheres that could be incorporated is 10 wt %. At this loading, the slurry mixed well and also set and hardened well. At 15 wt %, the setting was only marginal, and at 20 wt %, the volume of the extendospheres was so large that they separated from the slurry during pouring into the consistometer cup.
Also the light slurry started moving with the paddle during the test, and the consistency could not be measured. For these reasons, we conclude that 10 wt % extendospheres is the maximum loading that is possible for a rugged composition of the cement.
It is possible to estimate theoretically the effect of the extendosphere content on the thermal conductivity of the cement. Theoretical models10 predict that, for a cement with x% concentration of the spheres the thermal conductivity drops by a factor (1 - x)y, where y is between 2 and 3.
The thermal conductivity of ceramicrete concrete is quite low, 0.27 w/mK . Its thermal conductivity will thus be 0.2 -0.22 w/mK. when 10 wt % extendospheres are added, and 0.17 - 0.19 w/mK. when 15 wt % extendospheres are added. These thermal conductivity values are among the lowest reported for any cement.
Increase in setting strength with wollastonite
Earlier studies8 have shown that the strength of ceramicrete mixtures depends on the ash composition. Class F ash contains carbon, whose concentration in ash varies depending on the coal and the burners used. Therefore, depending on the composition of this ash, the strength of the permafrost cement may vary.
To eliminate the uncertainty arising due to Class F ash, we replaced it with Vansil W-10 wollastonite (CaSiO3), that passed through 200 mesh. The composition of the slurry was similar to the third composition given in Table 1, except that wollastonite replaced Class F ash in the ash blend and MgO content was slightly less. Fig. 1 shows the variation in consistency over time for this slurry composition (Fig. 1b) and for a slurry made with Class F ash (Fig. 1a).
The initial Bearden consistency is lower in the composition with wollastonite compared to that with ash, and the pumping time is slightly longer. The time of thickening was 5 hr, 45 min for the wollastonite composition as compared to 3 hr, 25 min with ash. The low Bc and longer thickening time must be due to slightlyless MgO in the wollastonite composition as compared to ash.
As before, when the samples with wollastonite were cured in a subzero environment in a freezer for 10 hr, the compressive strength was 14 MPa.
The results shown in Fig. 1 indicate that wollastonite is preferable over Class F ash because wollastonite cures the permafrost cement faster and also contributes to higher strength. Detailed studies are under way to produce superior permafrost cements with wollastonite and will be reported.
There are several reasons for the better performance of the ceramicrete slurry with wollastonite. Wollastonite particles have an acicular structure, and hence the resulting product has superior mechanical properties. The acicular grains help to deflect cracks and thereby increase the fracture energy. Furthermore, partial dissolution of calcium silicate grains introduces calcium ions in the slurry, which helps in forming calcium phosphate binder phases.5
Discussion
Tables 1 and 2 present the curing times and compressive strength under permafrost conditions for a ceramicrete composition and other cements reported in literature.
Comparison of the data indicates that ceramicrete blend is the most suitable candidate for oil field applications in permafrost wells. Other cements either do not develop sufficient strength or, if they do, do not provide sufficient pumping time.
Ceramicrete, combined with fly ash or wollastonite, on the other hand, gives sufficient pumping time, exhibits reasonably good strength, is unaffected by downhole gases, and is a good insulator. Its properties can be tailored to particular applications by partially replacing the ash by suitable extenders and varying their composition.
Unlike conventional cement, ceramicrete binder is rapid-setting due to an acid-base reaction that is characteristically exothermic. This exothermic heat could affect the surrounding permafrost formation during the application of the cement. Therefore, we estimated the heat generated during formation of the ceramicrete by substituting the enthalpies of formation5 for each of the components in the equation. Table 3 presents the literature values of the enthalpies along with the calculated heat of reaction.
Taking the value of -123 kJ/mole (1,000 J/mole) of exothermic heat produced and considering that the molar weight of MgKPO4·6H2O is 266.4 g, we calculated that 462 J of heat will be generated per gram of MgKPO4·6H2O. If the slurry composition is 40 wt % binder and 20 wt % water, with the rest being unreactive filler, the heat generated will be 60% of 462 J, which is 277 J/g or 119.4 btu/lb of the slurry.
Maier et al.1 tested lumnite and “ciment fondu,” which are calcium aluminate cements with 50 wt % fly ash, and found that the heat of hydration was 1.27x105 and 2.06 x105 J/kg (57 and 92 btu/lb) of the slurry, respectively. Our theoretical value is higher than these measurements. Lumnite and ciment fondu, however, did not set at subzero temperature, while ceramicrete-based cements did.
Moreover, the calculated heat of formation for ceramicrete cement is not excessively high, and its thermal conductivity is very low. Therefore, it may be possible to use ceramicrete cement without thawing the surrounding formation.
Alternatively, increasing the amount of unreactive filler in the formulation could reduce the heat of formation. Such investigations should be conducted in actual yard or field tests.
In this article, we have addressed use of the ceramicrete cement for drilling and completion of oil and gas wells. Because of the superior strength, very low permeability, and very low thermal conductivity, use of this material may be extended to construction of pipeline supports, insulating cement in cold regions, and other civil engineering applications in cold climates.
Acknowledgment
This work was supported by the US Department of Energy, National Petroleum Technology Office and National Energy Technology Laboratory, under Contract W-31-109-38. ✦
References
1. Maier, L.F., Carter, M.A., Cunningham, W.C., and Bosley, T. G., “Cementing Materials for Cold Environments,” J. Petroleum Technology, October 1971, pp. 1215-1220.
2. Morris, E.F., Evaluation of Cement Systems for Permafrost, SPE Preprint No. 2824, 1970.
3. Benge, O.G., Jones, R.R., Dresher, T.D., and Dolan, R. T., A New Low-Cost Permafrost Cementing System, SPE Preprint No. 10757, 1982.
4. American Petroleum Institute Standards, Spec.10.
5. Wagh, A.S., Chemically Bonded Phosphate Ceramics, Elsevier Science (UK) BV, 2004.
6. Jeong, S.Y., and Wagh, A.S., “Cementing the Gap between Ceramics, Cements, and Polymers,” Materials Technology, Vol. 18 (2003), No. 3, pp. 162-168.
7. American Association of State Highway and Transportation Officials, Laboratory Evaluations of Rapid Set Concrete Patching Materials, Report NTPEP 160, December 1999.
8. Wagh, A.S., Jeong, S.Y., and Singh, D., “High Strength Phosphate Cement using Industrial Byproduct Ash,” Proc. 1st Conference on High Strength Concrete, Amer. Soc. Civil Eng., 1999, pp. 542-555.
9. Smith D.K., Cementing, SPE Monograph 4, 1990, p.190.
10. Wagh, A.S., “Porosity Dependence of Thermal Conductivity of Ceramics and Sedimentary Rocks,” J. Mater. Sci., Vol. 28 (1993), pp. 3715-3721.
11. Goode, J.M., “Gas and Water Permeability Data for Some Common Oil Well Cements,” J. Petroleum Technology, August 1962, pp. 851-854.
The authors
Shirish Patil ([email protected]) is an associate professor of petroleum engineering at the University of Alaska Fairbanks. He holds an MS in mechanical engineering from the University of Pittsburgh, an MS in petroleum engineering and engineering management from the University of Alaska Fairbanks, and a BE in mechanical engineering from the University of Pune, India. Patil’s research interests include gas hydrate phase behavior, production modeling, miscible and thermal enhanced oil recovery processes, CO2 sequestration, and gas-to-liquids transportation. He is a member of SPE.
Editor’s note: Biographies and photographs of the other three authors appeared last week (OGJ, May 9, 2005, p. 53).