Lisbon gas plant installs acid-gas enrichment, injection facility
LISBON GAS PLANT—1
Tom Brown Inc. completed construction of an acid-gas enrichment (AGE) and acid-gas injection (AGI) facility at its Lisbon gas plant near Moab, Utah, in May 2003. Lean acid-gas feed is treated with ExxonMobil Research & Engineering Co.'s Flexsorb SE amine solvent to produce an enriched acid gas.
This first part of a two-part series on the Lisbon plant discusses the acid-gas enrichment portion of the facility in detail, with an emphasis on the Flexsorb treating unit. This article discusses the design of AGE-AGI facility, illustrating key issues with 30 days of hourly operating performance data.
Incremental benefits associated with the AGE-AGI facility were sufficient to justify the capital investment, which was about $4.2 million, or $2/Mcf of H2S removed during the project's lifetime.
Capital investment was reduced significantly because an ideal AGI well was available at a low cost. In addition, much of the AGE facility was scavenged from various parts of the Lisbon plant's sulfur-recovery unit and retrofitted for new service.
The conclusion next week will discusses the design and operation of the compression and injection portions of the facility.
Lisbon gas plant
The plant lies about 40 miles south of Moab in southeast Utah. Built in 1962 by the Pure Oil Co., it consisted of oil stabilization and gas compression. Associated sour gas (60 MMcfd) was reinjected into the gas cap of the volatile oil reservoir for pressure maintenance.
NGL recovery and fractionation facilities were added in 1966. Lean processed sour gas continued to recycle to the reservoir for pressure maintenance for the next 27 years.
Gas sweetening and conditioning, nitrogen rejection, and helium-recovery facilities were installed in 1992, with gas cap blowdown and sale of residue gas starting in September 1993.
Unocal Corp. operated the plant until July 1999 when its Rocky Mountain assets were acquired by Tom Brown Inc., Denver, an independent exploration, production and drilling company that focuses on gas resources in the Rocky Mountains (including Canada) and Texas.
Alternative to Selectox
Starting in September 1993, H2S entering the plant was catalytically converted to liquid sulfur in a Selectox sulfur-recovery unit (SRU). This SRU successfully operated for 7 years (OGJ, Aug. 27, 2001, p. 44) but with high operating costs.
In early 2002, the cost of sulfur disposal had increased significantly and catalyst in the reactors was approaching end of run. Another catalyst change would cost well more than $1 million.
Tom Brown authorized a scoping study starting in February 2002 to find a cheaper and better way to dispose of H2S entering the plant. Washington Group International Inc. was selected to work with Tom Brown's engineering staff due to Washington's experience with sulfur recovery and acid-gas injection.
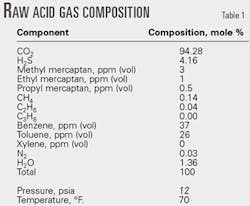
All the H2S entering the plant is absorbed in the methyldiethanolamine (MDEA) treating unit and concentrated into the "MDEA raw acid gas," which feeds the SRU. Design flow rate of the MDEA raw acid-gas feed is 14 MMcfd containing about 4% H2S, 94% CO2, and 2% water.
The scoping study showed that the best alternative to the SRU included enrichment of the raw acid gas from the MDEA regenerator. This would concentrate the H2S entering the plant into a much smaller flow stream. Enriched acid gas is injected into a disposal well. The combination is called AGE-AGI.
null
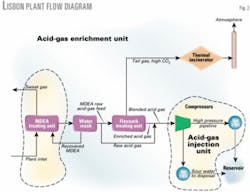
The AGE facility (Fig. 1) produces an enriched acid-gas product stream as small as 1 MMcfd containing about 95% of the H2S but only 4% of the CO2 that enters the facility.
The H2S content of the enriched acid gas is concentrated to >50% by volume (plus 50% CO2). Enriched acid gas flows to the AGI facility where it is compressed from 10 psig to about 1,500 psig, then transported in a pipeline for injection down a wellbore into the reservoir from which it originated.
Tail gas (mostly CO2) remaining after the H2S is removed from the MDEA raw acid-gas feed flows to a thermal incinerator where it is released to the atmosphere without compression in full compliance with the plant's air quality permit.
Tom Brown was fortunate to have an existing tail-gas treating unit (part of the SRU) and most of its equipment was suitable for conversion into service for the AGE unit at a substantial discount to the cost of building a grassroots facility. Tom Brown was also fortunate to have a sour-water disposal well, which was ideal for conversion to AGI service.
The AGI well and reuseable process equipment reduced the total capital investment requirements for the proposed AGE-AGI facility.
AGE-AGI vs. existing SRU
An economic comparison between the AGE-AGI process and continued operation of the existing SRU indicated that lower operating costs and increased production with the AGE-AGI process provided sufficient economic benefit to justify the capital investment.
The attached box lists key economic variables relevant to this decision. Part of the savings was eliminating costs to dispose of pure molten sulfur produced by the SRU (21 long tons/day). Additional savings were attributed to eliminating the cost of future catalyst replacements for the SRU reactors.
Continued SRU operation would require several million dollars to replace aged and spent catalyst during the remaining life of the plant.
The AGE-AGI process would also increase production in three ways: reduce fuel gas consumption, improve operating run time, and extend plant economic life.
The project proposal was presented to Tom Brown's senior management on June 19, 2002, and approved the same day. The project was completed and fully operational 11 months later on May 10, 2003.
AGE facility
Fig. 2 shows a block flow diagram of the AGE-AGI facility. Recently, inlet well stream gas production to the plant averaged about 50 MMcfd of sour natural gas containing 27% CO2, 1.1% H2S, 15% nitrogen, 0.77% helium, and about 56% hydrocarbons.
The CO2 and H2S are removed in the MDEA treating unit, which currently yields 10-12 MMcfd of MDEA raw acid-gas feed. Specialized on site gas-sample analysis identified trace compounds in the MDEA raw acid gas. These included light hydrocarbons, aromatic hydrocarbons, various sulfur compounds, CO2, and H2S (Table 1).
null
The facility's AGE portion includes everything between the MDEA treating unit and AGI unit.
Most of the MDEA raw acid-gas feed flows to the Flexsorb treating unit. Some gas, however, bypasses the treating unit and flows directly to the injection compressors in the AGI unit to maintain constant suction pressure.
Bypassed acid gas mixes with the enriched acid gas exiting the treating unit.
This blended acid gas flows to the injection compressors.
Flexsorb treating unit
ExxonMobil provided process design for the treating unit as part of the licensing agreement. The process design specification calls for a conventional amine treating configuration, supplies energy and material balances, and includes detailed recommendations for all equipment.
The severely hindered amine used in the Flexsorb solvent is specifically tailored to give high H2S absorption capacity in the presence of CO2. This property allows the solvent to achieve a high H2S cleanup and selectivity at low solvent circulation rates.
Absorber
The Flexsorb absorber is a thin-wall (1/4 in.) carbon steel vessel, 6.5 ft diameter and 48 ft long seam-to-seam, that has been fitted with 12 conventional valve trays. Three alternate feed nozzles are on Trays 5, 7, and 9.
The absorber is internally coated with a 1/4-in. thickness of Ashland Chemical Co.'s Hetron 980 fiberglass. Maximum allowable temperature of the fiberglass coating is 175° F.
The heat of absorption of acid gas into the solvent causes a temperature increase of about 20-30° F. in the rich solvent leaving the absorber.
The maximum bulge temperature in the absorber occurs on an intermediate tray and is about 15° F. higher than the outlet rich solvent.
The maximum expected bulge temperature is about 155° F.
The absorber was previously a tail-gas cleanup absorber containing 10 ft of structured packing. It was retrofitted with 12 valve trays and internally coated for about $130,000.
The absorber normally operates at 8 psig.
The operating pressure was varied 6-15 psig in an effort to improve H2S removal efficiency. No significant performance improvement occurred during these changes.
The air coolers control the lean-solvent temperature and MDEA raw acid-gas feed temperature; therefore, they vary with ambient conditions.
The lean-solvent feed flow rate to the absorber was relatively constant at about 160 gpm from start-up until late September 2003, when it increased to about 175 gpm.
The lean-solvent circulation rate was reduced to 150 gpm when the weather turned cold in November.
Regenerator
The Flexsorb regenerator (Fig. 3) is a new 1/2-in. wall carbon steel vessel that is 4.5 ft diameter, 80 ft long seam-to-seam, and contains 28 stainless steel conventional valve trays.
During start-up, the the new solution turned from clear yellow to dark black immediately after acid gas was introduced to the unit.
Iron sulfide particles that formed during the passivation of the carbon steel surfaces in the regenerator and carbon steel piping caused the black color.
The solution returned to clear yellow after dozens of sets of sock-filter elements (10 mm and larger) removed the suspended solids. The sock filters initially lasted less than 1 hr but now last a couple of months.
The installed cost of the new regenerator next to the existing sulfur tail-gas unit was $120,000.
The regenerator overhead pressure averaged 17 psig and about 225° F. These operating conditions resulted in a reflux ratio of 2.0 mole water/mole acid gas.
Regenerator bottoms temperature is about 260° F. and the rich-solvent feed temperature is about 210° F.
The regenerator reflux accumulator collects solids and black gunk; reflux water is filtered at the reflux pump discharge.
Some of the reflux stream can flow to the closed drain to purge water from the system.
H2S recovery efficiency
H2S recovery efficiency of the Flexsorb treating unit is the percentage of H2S entering the absorber that is recovered in the enriched acid gas flowing to the suction of the injection compressors.
H2S not captured by the solution is subsequently oxidized and vented to the atmosphere through a thermal incinerator.
MDEA raw acid-gas feed that bypasses the absorber directly to the compressor suction is not included in the H2S-removal efficiency calculation.
The concentration of H2S in the inlet MDEA raw acid-gas feed is measured with a continuous online chromatograph and was relatively constant at 4.6%.
A continuous online chromatograph also measures the S02 concentration in the stack gas leaving the thermal incinerator.
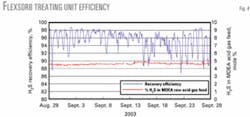
H2S recovery efficiency is 90-98% (Fig. 4). One can more conveniently estimate H2S-recovery efficiency by examining the mass of H2S vented to the atmosphere from the thermal incinerator after it oxidizes to form S02.
For example, S02 emissions of 300 lb/hr equate to about 91% recovery efficiency, while 100 lb/hr equate to about 97% recovery efficiency.
Absorber operating temperature
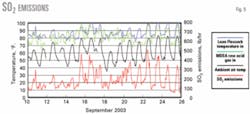
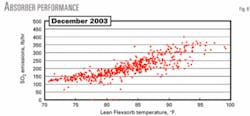
Fig. 5 shows lean solvent, MDEA raw acid-gas feed, and ambient air temperatures vs. S02 emissions. Variations in ambient air temperature cause changes in the temperature of the lean solvent and MDEA raw acid-gas feed to the absorber, which causes changes in S02 emissions.
Due to the obvious relationship between these variables we prepared an additional plot. Fig. 6 shows S02 emissions vs. lean solvent temperature.
The H2S recovery efficiency of the treating unit, as indicated by S02 emissions, definitely increases as the temperature of either the lean solution or MDEA raw acid-gas feed decreases.
The temperature of the lean Flexsorb solution has a stronger correlation with H2S recovery efficiency than the MDEA raw acid-gas feed temperature.
There is significant scatter in the data that indicates that other factors must also be working to influence H2S recovery efficiency.
Directionally, lower temperatures should improve H2S selectivity, reduce CO2 coabsorption, reduce enriched acid-gas volume, and increase enriched acid-gas H2S concentration.
The operation of air coolers with local temperature controllers determines the temperature of the lean solution and MDEA raw acid-gas feed. Lisbon plant operators intentionally allowed the lean Flexsorb temperature to swing with ambient temperature. During summer months, they keep the temperature as low as possible during the 24-hr daily cycle.
The daily temperature swing is about 20° F. with a September 2003 low of about 80° F. and a December 2003 low of about 70° F.
The lean solvent temperature could be controlled more tightly if more-sophisticated controllers or variable-speed fan motors on the air coolers were installed.
The operators do not allow the lean solvent temperature to fall below 70° F. because the solvent's viscosity increases and treating effectiveness might decrease below this minimum temperature.
Treating unit design basis
Tom Brown asked ExxonMobil to develop a process basis for the treating unit so that S02 emissions, on average, do not exceed 300 lb/hr; this is below the fixed cap on Lisbon plant emissions according to the Title V air quality permit.
CO2 pickup should be minimized but a specific target was not specified. It is clear from Fig. 5 that the treating unit is performing better than expected; in fact, S02 emissions average much less than 300 lb/hr.
When the lean solution temperature is low (80° F.), S02 emissions average 150 lb/hr (Fig. 6), which is equivalent to 96% H2S recovery efficiency.
Conversely, Fig. 5 shows that when the operators cannot control the lean solution temperature during the hot summer, S02 emissions will rise to more than 300 lb/hr during the afternoon hours.
During start-up in May 2003, the top feed (Tray 5) to the absorber was lined up for service and the S02 emissions were so low (less than 50 lb/hr) that the plant operations staff thought the chromatograph on the thermal incinerator was malfunctioning.
H2S recovery was exceptional using the top feed nozzle; however, with the extra H2S came extra CO2 to the point that the increased flow exceeded the capacity of the injection compressors.
We therefore had to change to the lowest feed nozzle (Tray 9) on the absorber; it has been in service ever since.
The lowest feed nozzle basically takes the four trays above it out of service, leaving four active trays below. It makes sense that adding or subtracting fractionation trays or stages will change the H2S and CO2 pickup by the solution.
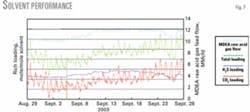
Rich-solution loading
Fig. 7 shows loading of the solution in terms of moles of H2S/mole of solvent and moles of CO2/mole of solvent. MDEA raw acid-gas feed flow rate is shown for reference.
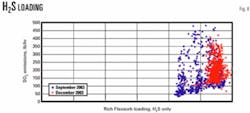
Fig. 8 shows rich solution H2S loading vs. S02 emissions. One evident trend is that H2S loading occurs in a narrow range.
Another trend is that H2S rich loading was higher in December 2003 than in September 2003, presumably due to lower temperatures.
Each mole of Flexsorb solvent can hold more H2S at lower temperatures. This observation supports the previous assertion that directionally, lower temperatures should improve H2S selectivity and increase enriched acid-gas H2S concentration.
As the rich CO2 loading of the Flexsorb solution increases, the H2S recovery efficiency decreases.
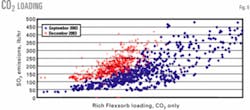
Fig. 9 shows rich CO2 loading data in September and December 2003. Solution-rich CO2 loading is clearly lower in December 2003 than it was September 2003 despite the fact that the lean-solution circulation rate was lower in December.
The lower CO2 loading in December, therefore, must be related to temperature. The daily cycle in CO2 loading in Fig. 7 is obviously related to ambient temperature swings. Rich-solution CO2 loading changes daily and seasonally with temperature.
This observation supports the previous statement that directionally, lower temperatures should reduce CO2 coabsorption, reduce enriched acid-gas volume, and increase enriched acid-gas H2S concentration.
Fundamentals of AGE unit performance
In cooler weather when the treating unit's temperatures are low, the amount of H2S that the Flexsorb solution absorbs increases (Fig. 8) and CO2 absorption decreases (Fig. 9).
This results in higher H2S concentration and lower molar volume of enriched acid gas from the regenerator that flows to the injection compressors.
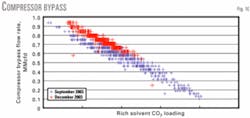
Less flow of enriched acid gas from the regenerator causes more MDEA raw acid-gas feed to bypass the treating unit to maintain a constant compressor suction pressure.
More MDEA raw acid-gas feed bypassing the absorber decreases the feed gas flow into the absorber. The absorber's H2S-recovery efficiency increases because there is less total feed-gas flow with a constant lean Flexsorb solution flow.
According to the Kremser-Brown correlation, V (vapor molar flow) decreased but L (liquid amine molar flow) remained constant; therefore, L/V increased, which causes treating efficiency to improve.
This conclusion is supported by a plot of rich-solvent CO2 loading vs. MDEA raw acid-gas flow that bypasses the treating unit (Fig. 10).
Rich solution held less CO2 in December 2003 than in September 2003, which resulted in considerably more flow bypassing the treating unit.
Based on a presentation to the 54th Annual Laurance Reid Gas Conditioning Conference, Feb. 22-25, 2004, Norman, Okla.
The authors
Steven G. Jones is a senior chemical engineer at Tom Brown Inc.'s Lisbon gas plant, Moab, Utah. During his 10-year tenure at the plant, he has participated in start-up, troubleshooting, and operations of the amine treating, sulfur recovery, NGL recovery and nitrogen rejection, and helium purification, and liquefication units. In 1999, Jones was assigned additional duties as project manager and project engineer for new facilities in Utah and Colorado. Previous to Lisbon, he worked for 15 years in petroleum reservoir and production engineering for Unocal Corp., Mobil Oil Corp., and Mesa Petroleum Corp. Jones holds a BS (1978) in chemical engineering from the University of New Mexico and is a registered professional engineer in Colorado.
Doug Rosa is plant manager at Tom Brown Inc.'s Lisbon gas plant, Moab, Utah. During the past 5 years, he has been responsible for managing all phases of the Lisbon plant. Before Tom Brown acquired the plant from Unocal Corp. in 1999, Rosa worked there for 5 years as a measurement technician and engineering assistant on various projects. Previous to that, he worked for 2 years on an offshore production platform in the Gulf of Mexico, 3 years at Unocal's shale oil project near Parachute, Colo., and 5 years for various drilling contractors in Colorado, Wyoming, Utah, and California. Rosa holds an associate degree (1982) in petroleum technology from Casper College, Wyo., and attended Nichols State University's (1992) petroleum technology program.
Johnny E. Johnson is a technology manager for Washington Group International Inc., Denver. He consults with project managers, lead engineers, and discipline engineers working on gas conditioning and sulfur-related projects. He also frequently serves as technical consultant and lead engineer for special projects. He holds a BS in chemical engineering from the University of Colorado. Johnson has served as chairman of a research committee of GPA and is a member of the advisory board for the Laurance Reid Gas Conditioning Conference.