Correlation estimates FCC unit sulfuric acid emissions
A new correlation estimates the amount of sulfuric acid emissions in FCC flue-gas streams based on the amount of SO2 formed in the unit. The correlation focuses on the SO2 to SO3 conversion expected in FCC units.
FCC units are a potential source of H2SO4 emissions, but few published sources contain a reliable estimate of these emissions.
Based on the need to better understand all sources of H2SO4, we analyzed data collected from various field tests to estimate SO2 and SO3 conversions. We compared SO2 and SO3 measurements and developed a correlation for predicting the conversion as a function of sulfur dioxide. This article also presents data on scrubber control efficiency for H2SO4.
One can obtain the amount of SO2 formed from a mass balance from fuel sulfur or stack monitoring and testing. SO3 to H2SO4 conversion is based on a process model.
Sulfur oxides (SOx) are generated in combustion devices from the oxidation of sulfur contained in the fuel. SOx emissions from combustion devices are predominantly SO2. A small portion of SO2 further oxidizes to SO3. Within certain temperature ranges, SO3 readily reacts with water vapor to form H2SO4.
Some regulatory agencies require that plant owners report H2SO4 emissions. In the US, Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) of 1986 requires facilities that manufacture, process, or otherwise use listed toxic chemicals above specific threshold quantities report the environmental release of those chemicals annually.
Sulfuric acid in any airborne form (aerosols, mists, vapors, gas, fog) is on this list, which means that its release is subject to reporting if the reporting threshold is triggered.
Background
In June 1995, the US Environmental Protection Agency modified the guidance for sulfuric acid reporting requirements by deleting non-aerosol forms of sulfuric acid under Section 313 of EPCRA.1
The current listing, however, defines "aerosol" forms rather broadly. It reads: "Sulfuric acid aerosols [include] mists, vapors, gas, fog, and other airborne forms of any particle size."
Subsequently, in November 1997, EPA issued its "Guidance for Reporting Sulfuric Acid," which discusses sulfuric acid emissions from industrial sources.2 This guidance was updated in March 1998.
The 1997 EPA guidance stated that the combined SO3 and H2SO4 product from combustion sources is between about 1% and 3% of the total SOx. The guidance appears to imply that this conversion applies to all fuels (gas, liquid, and solid) irrespective of the types of combustion devices or sulfur content.
New test data have become available since this guidance was published that indicate SO2-to-SO3 conversion is affected by both fuel type and sulfur content (OGJ, Sept. 30, 2002, p. 78).
Papers that focused mainly on SOx control options have reported limited data on expected conversions of SO2 to SO3 for FCC units. Researchers that attempted to model the equilibrium found that the SO3 concentration increases rapidly at higher oxygen inputs (OGJ, Feb. 23, 1981, p. 55). These authors found conversion rates of about 1% of the SO2 to SO3 based on flue-gas composition measurements.
As part of a study to develop a desulfurization catalyst, Byrne proposed typical conversion numbers for sulfur.3 This author recommended the assumption that 5% of the feed sulfur was deposited on the catalyst and subsequently burned off in the regenerator.
The recommended assumption for the relative amounts of SO2 to SO3 in the flue gas was a ratio of 9:1, equating to a 10% conversion. The author, however, did not include any data that supported these assumptions.
Subsequent papers repeated similar assumptions but supporting flue gas measurements were still not reported.4 5
Sulfuric acid formation
When a sulfur-bearing fuel is burned, essentially all of the sulfur is initially converted to SO2, which is the primary form of sulfur emissions from combustion equipment.
SO2 has a strong tendency to react with oxygen to form SO3 thermodynamically; but the reaction rate is slow in the gas phase under normal conditions. Once SO3 forms, however, it readily converts to H2SO4 (within milliseconds) at normal humidities and within certain temperature ranges. The determination of percent conversion of SO2 to SO3 is therefore critical when one estimates H2SO4 formation in FCC units.
The chain reaction occurs via these reactions:
S+O2 → SO2
SO2+1/2 O2 ↔ SO3
SO3+H2O ↔ H2SO4
Conversion from SO2 to SO3 during the combustion process is a complex function of fuel type, sulfur content, excess oxygen, flue-gas temperature, the catalytic effect of fuel-contained trace metals (e.g., vanadium, sodium, etc.), and the type of combustion unit.
Regenerator without scrubber
FCC regenerators are one of two combustion types, generally known as full-burn and partial-burn regenerators.
In a full-burn unit, coke is combusted completely to CO2, and the gas exits the regenerator with an excess of combustion oxygen. SOx and NOx constitute most of the emissions due to excess oxygen.
In a partial-burn unit, not all the coke is converted to CO2, leaving substantial amounts of CO in a reducing environment in which SOx remains the predominant sulfur species. NH3 and HCN comprise the predominant nitrogen species. Refineries typically combust remaining CO to CO2 in a downstream CO boiler or furnace, essentially converting all CO to CO2.
Based on a new analysis, we developed an equation that predicts conversion of SO2 to SO3 for FCC units. The analysis included recently tabulated data from previous field tests at the wet gas scrubber inlet and data from American Petroleum Institute document 4713.6
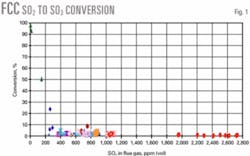
Fig. 1 shows all the data. Conversion of SO2 to SO3 is rather low except at low sulfur contents—less than 300 ppm (vol) SO2.
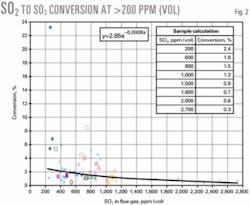
Fig. 2 redisplays the results without the data below 200 ppm (vol) SO2.
Table 1 summarizes the mean and median conversions of SO2 to SO3 in different SO2 ranges.
Conversion of SO2 to SO3 in an FCC unit with more than 200 ppm (vol) SO2 is generally 1-2%. An exponential correlation provides the best fit for these data and results in a prediction that is close to the mean.
We recommend this correlation to predict SO3 from FCC units:
y = 2.85e-0.0008x
where:
y = Conversion SO2 to SO3, %
x = Flue gas SO2 content, ppm (vol)
The correlation is applicable for SO2 concentrations above 200 ppm (vol) and for units without any downstream control equipment such as wet gas scrubber. A box in Fig. 2 shows sample results using the correlation for selected SO2 levels.
Regenerator with wet gas scrubber
Facilities equipped with a wet gas scrubber to control particulate and SOx emissions have about 4.6% of the measured scrubber outlet SO2 converting to SO3 based on limited stack tests at one location.
This factor is based on six "Method 8" samples taken on 2 days about 6 months apart. The measured conversion to SO3 was 2-8% with scrubber outlet SO2 concentrations of 15-37 ppm (vol).
There is insufficient data to confidently determine if there is any trend in SO3 conversion with feed sulfur. Due to limited data and concerns about the reliability of Method 8, we believe that site-specific source test data would likely provide a more representative estimate.
One can use the 4.6% conversion within the scrubber outlet SO2 concentration range if no other data are available. Assume that 100% of the predicted SO3 goes to H2SO4 due to high moisture and low temperature at the scrubber stack.
An API research program should generate additional data. In the future, the recommended emission estimating procedure may change based on the findings of the planned study or other information that may become available.
SO3-to-H2SO4 conversion
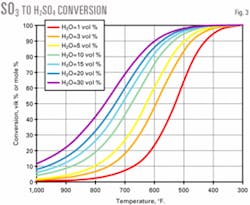
Fig. 3 shows the conversion of SO3 to sulfuric acid as a function of flue gas moisture and temperature. We assumed that the H2SO4 formation reaction (H2O (g) + SO3 (g) → H2SO4 (g)) reaches equilibrium.
The attached box shows two sample calculations, one for an FCC unit without a wet gas scrubber and one with a wet gas scrubber.
References
- "Sulfuric Acid: Toxic Chemical Release Reporting: Community Right-to-Know," Final Rule, 60 FR 34182, US Environmental Protection Agency, Washington, June 30, 1995.
- "Emergency Planning and Community Right-to-Know Act - Section 313. Guidance for Reporting Sulfuric Acid (acid aerosols including mists, vapors, gas, fog, and other air borne forms of any particle size)," EPA-745-R-97-007, US Environmental Protection Agency, Washington, November 1997.
- Byrne, J.W., "New Developments in FCC SOx Catalyst Technology," presented to the NPRA Annual Meeting, paper AM-84-55, Mar. 25-27, 1984, San Antonio.
- Wall, J.D., "Control FCC SOx Emissions," Hydrocarbon Processing, October 1984, pp. 45-46.
- Evans, M., "SOx Reduction," Hydrocarbon Engineering, January 2003, pp. 43-44.
- "Test Report: Fluidized Catalytic Cracking Unit at a Refinery (Site A)—Characterization of Fine Particulate Emission Factors and Speciation Profiles," API Publication 4713, American Petroleum Institute, Washington, DC, March 2002.
The authors
W. X. Nie is a senior engineer with ExxonMobil Research and Engineering Co., Fairfax, Va. Currently, he is involved in emissions release estimations and preparation of Title V permits. At ExxonMobil, Nie initially focused on heat exchanger design and troubleshooting, process integration, and combustion simulation. Before joining ExxonMobil, he spent 3 years as an energy engineer in the pulp and paper industry. He holds a BS in chemical engineering from Tsinghua University, China, and a PhD in chemical engineering from the University of Manchester, UK.
J. H. Siegell is a senior engineering associate with ExxonMobil Research & Engineering Co. where he heads activities on estimating and controlling air toxic, hydrocarbon, and odorous emissions. He has more than 25 years' industrial experience in fundamental and applied research, and technical support to refineries and chemical plants. Siegell holds a BE and ME in chemical engineering from the City College of New York and a PhD in chemical engineering from the City University of New York. He is active on several API committees and currently leads the API smart leak detection and repair (LDAR) program.
M. S. McWilliams is the environment and global compliance manager for ExxonMobil Refining & Supply Co., Fairfax, Va., where he heads a group responsible for federal regulatory advocacy, compliance support, and worldwide compliance assessment activities for the downstream and chemicals divisions. He has 30 years' experience in refining and supply, including technical and operating supervisory and management responsibilities at the Baytown, Tex., and Baton Rouge, La., refineries and headquarters functions. He holds a BS in chemical engineering from Texas A&M University. He is active in API.
T. P. Yarnick is a senior environmental advisor with ExxonMobil Refining & Supply Co., Houston, where he supports both the petroleum and chemical manufacturing businesses. He has more than 25 years' experience with ExxonMobil in the environmental and regulatory compliance arena and is currently leading a water and waste team responsible for regulatory advocacy at the federal level and providing compliance support to more than 30 operating facilities. His areas of specialization include Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and Emergency Planning and Community Right-to-Know Act (EPCRA) release reporting and right-to-know issues. He holds a BS in environmental biology from the University of Pittsburgh.
O. Biceroglu is the environmental management and performance reporting manager with Imperial Oil Ltd., Products and Chemicals Division, Toronto, Ontario. He has over 25 years' experience with Imperial Oil that covers refining research and development, environmental management, regulatory compliance, and emission inventory development and reporting processes. Biceroglu holds a BSc from Middle East Technical University, Ankara, Turkey, an MSc from the University of Tulsa, Okla., and a PhD from McGill University, Montreal, all in chemical engineering.