Despite the wide disparity of views among experts about remaining recoverable hydrocarbon resources and the timing of peak production of conventional reserves, certain trends are not in dispute:
Crude liquid petroleum production will yield increasingly heavy crudes.
The demand for natural gas will increase faster than the demand for other energy resources.
A process able to extend hydrocarbon supply by capitalizing on these trends is conversion of petroleum resid or heavy crude oil to methane by reaction with steam. The process discussed here is suitable for conversion of any heavy hydrocarbon liquid feed, including atmospheric or vacuum resids, FCC or coker fractionator bottoms, steam cracker tar, deasphalter rock, and Canadian or Venezuelan bitumens. The main requirements are that the liquid be substantially free of inorganic contaminants (less than 0.1%) and that it be fluid at a temperature below about 800° F.
The reason for a catalytic approach is the much higher conversion efficiency of about 85% vs. about 50% for conventional oxygen-based conversion.
Resid's real value
Resid as discussed here is generally the bottoms product from vacuum distillation, typically having an atmospheric boiling point equivalent to 1,050° F. or higher (1,050º F.+). It is thus the nondistillable fraction of crude oil. It is important to distinguish this fraction from residual fuel, a blend of resid and distillate formulated to meet viscosity and sulfur limits required for direct combustion. Residual fuel is commonly referred to as "resid" but must not be confused with 1,050° F.+ resid.
Various approaches are used for evaluating resid as a fuel or feed to conversion processes, generally by valuing the end products and cost of conversion to arrive at a netback value for the starting resid. Indeed the current major disposition of resid is delayed coking, a thermal decomposition which yields less than 60% low-quality liquids. Coker liquids require significant upgrading by hydrotreating to meet fuel-quality specifications. Since the resid value tends to be low relative to the products, variations in estimates of conversion efficiency and costs result in wide variations in the estimated resid value. In fact there are no industry standard specifications for coker feed, and there are resulting legal issues around the computation of transportation tariffs.1
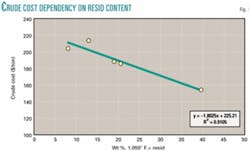
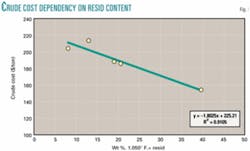
A less ambiguous approach is to value the resid at cost as if it were a blend component of crude oil. Crude oil is typically traded at prices that reflect gravity and sulfur. Gravity is highly correlated with resid content because the resid fraction has higher density than the distillable fractions. Landed costs reported by the US Energy Information Administration for five selected crudes are listed in Table 1 and plotted in Fig. 1 as $/ton vs. wt % resid.2
The correlation coefficient indicates that 91% of the variability in crude price is related to resid content, and the trend line shows the cost of resid as a component of crude oil in 2003 to be about $45/ton, while the distillate fraction (less than 1,050° F. atmospheric boiling point equivalent, or 1,050º F.–) cost is about $225/ton.
This interpretation does not separate variables such as sulfur content and viscosity and therefore represents an "average" resid. With sufficient data a similar analysis, which separates quality variables, would be expected to show lesser values for resids with high density, viscosity, sulfur, and nitrogen. At $45/ton for resid, its value is about $1.25/MMbtu, consistent with its competition with coal, and only 25-35% of the value of methane as natural gas.
Why methane?
It may seem anomalous to consider conversion of liquids to gas at the same time as other technology is being readied to convert gas to liquids, but the fact is that the value of methane depends more on where it is than what it is. Gas-to-liquids processes are of interest where large stranded reserves of gas have no access to gas pipeline infrastructures. GTL projects are alternatives to LNG projects, which require large investments at the gas fields as well as at the terminals where LNG is gasified and delivered to the pipeline. Most refineries however, are already close to natural gas pipelines: The resid is already available where the gas is needed.
Many other options for resid conversion, such as electric power generation, power and steam cogeneration, low-btu gasification, synthesis gas generation, or hydrogen manufacture, require large base-load customers because the products must be used at the time they are generated. But methane is fully interchangeable with natural gas, and resid conversion to methane will always be a small fraction of the total natural gas supply, not enough to influence the market price for natural gas.
Chemistry
A typical vacuum resid is about 87% carbon. The chemistry of the desired reaction for carbon alone may be written
1) 2 H2O + 2 C.Æ. CH4 + CO2
This reaction can be made to occur in the presence of an alkali metal catalyst such as potassium at temperatures of 1,200º-1,400º F. It is the basis for the Exxon Catalytic Coal Gasification (CCG) process developed in the 1970s but never commercialized because of unfavorable economics. The high costs associated with capital investment for coal handling, catalyst makeup and recovery, and spent-solids disposal made it unable to compete with then-regulated natural gas.
Reaction 1 still has great merit because of its very high conversion efficiency. Most gasification processes are very endothermic because they make H2 and CO, but Reaction 1 is only very slightly endothermic. Consequently, it is unnecessary to use oxygen or air to burn part of the feed (partial oxidation or POX) as in typical gasification processes. The necessary heat can be provided by superheating the reactants in a gas-fired furnace after recovering heat from the raw product gas.
At the preferred reaction conditions of 1,300º F. and 500 psig, H2 and CO are present in the product gas, but their net formation is prevented by separating them from the product methane, mixing with the reactant steam, and recycling the mixture of steam, H2, and CO to the reactor.
For petroleum resid, the real overall reaction must take into account the presence of hydrogen, nitrogen, and sulfur. Nitrogen in the feed is converted quantitatively to ammonia in the high temperature reducing atmosphere in the presence of alkali metals. Likewise, sulfur is converted primarily to H2S with trace levels of COS. Allowing for these byproducts as hydrogen sinks, the typical atomic H to C ratio remaining in the resid hydrocarbon is about 1.33. Consequently, the desired overall reaction for most vacuum resids may be written
2) 1.5 CH1.33 + H2O.Æ CH4 + 0.5CO2
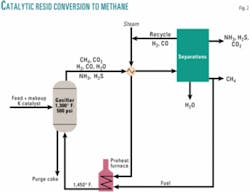
Resid or heavy crude upgrading processes are often classified as hydrogen addition, carbon rejection, or some combination of the two. Reaction 2 is the ultimate combination, with hydrogen addition supplied by steam, and carbon rejection as CO2. A schematic flow diagram is shown in Fig. 2. The gasifier reactor itself contains a captive inventory of fluidized solids comprising coke, potassium, and inorganic components present in the feed. The mixture of steam and recycled H2 and CO is preheated by exchange with the raw product gas and superheated in a gas-fired furnace to about 1,450º F. About 5% of the product methane is required by the superheat furnace.
The raw product gas contains methane, hydrogen, carbon monoxide, carbon dioxide, unreacted steam, ammonia, hydrogen sulfide, carbonyl sulfide, and entrained fine particles. The five main components have the composition shown in Table 2. After heat recovery down to about 40º F. above the dew point, the fines and ammonia are removed by wet scrubbing. H2S, COS, and CO2 are removed in a conventional acid-gas scrubbing process, and the remaining components, CH4, H2, and CO, are separated by cryogenic distillation.
About 10% of the product methane is used for cogeneration turbine fuel to supply power for the cryogenic refrigeration and feed steam. Overall, about 85% of the hydrocarbon heating value is converted to heating value of the net product methane delivered at a pipeline pressure of 1,000 psig.
Economy of scale will probably reach diminishing returns at about 100,000 b/d, requiring four 25,000 b/d reactor trains and two downstream trains for gas scrubbing, separations, and recycle.
Gasifier configuration
For the US patent application example, a commercial plant configuration was studied for the conversion of 25,000 b/d of a typical petroleum resid to methane using a feedstock with a specific gravity of 1.01 (8.9º gravity) and containing 4.1% sulfur, 0.1% nitrogen, and 0.01% inorganic components by mass.3
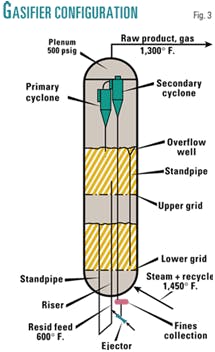
Conversion to methane by reaction with steam takes place in a two-stage gasifier shown schematically in Fig. 3.
The feedstock is stored at 300º F. in a heated and agitated storage tank. In the storage tank, extra-fine grade potassium carbonate (80% through 325 mesh) is added to the feedstock to a concentration of 0.1 wt %. The feedstock is preheated to a temperature of 600º F. in a heat exchanger and fed at about 550 psig through an array of four radially spaced feed injectors into the riser, a configuration commonly used in catalytic cracking. A portion of the steam and recycled hydrogen and carbon monoxide (about 5%) is also introduced through the feed injectors to atomize the liquid feed.
Solids are circulated through the standpipe and riser at about 1,000 lb/sec. The riser is aerated with about 5% of the steam and recycle gas below the feed injectors. Above the feed injectors, the ID of the riser is about 2 ft so that the circulating solids are transported upwardly at a velocity of about 20 fps, discharging into the upper stage. The liquid feed undergoes rapid thermal decomposition in the riser, yielding primarily hydrogen, methane, and petroleum coke. The petroleum coke is uniformly distributed as a coating on the entrained particles.
The gasifier is a refractory lined pressure vessel having an ID of about 30 ft, and having two fluidized bed stages, each having a depth of about 40 ft, supported by grids that allow the upflowing gases to pass through, fluidizing the solid particles. Solids inventory is controlled by monitoring the depth of the lower stage so as to maintain the overflow well inlet slightly submerged below the surface, ensuring a continuous supply of circulating solids, and withdrawing solids as necessary to allow a disengaging space below the upper grid.
The normal solids withdrawal rate will be about 900 lb/hr. The heating value of the petroleum coke in the withdrawn solids represents only about 0.2% of the heating value of the feed. The level of solids in the upper stage is controlled by an overflow well that discharges excess inventory into the lower stage. The total inventory of solids required is about 900 tons, having a composition of 53% petroleum coke, 43% potassium, and 4% other inorganic constituents by mass. The withdrawn solids are periodically analyzed to ensure that they are more than 50% coke, more than 30% potassium, and less than 10% other inorganic constituents. Withdrawal rates and catalyst addition rates may be adjusted to maintain the preferred composition.
The gasifier pressure is maintained at about 500 psig at the plenum by means of a back-pressure regulator located on the product gas line downstream of the heat-recovery and gas-scrubbing facilities.
About 90% of the steam and recycled hydrogen and carbon monoxide stream is preheated to about 1,100º F. by heat exchange with the raw product gas and superheated to about 1,450º F. in a gas-fired superheat furnace then fed to the gasifier below the lower grid. The actual outlet temperature of the superheat furnace is adjusted to control the gasifier temperature at the plenum at about 1,300º F.
Under these conditions, the composition of the feed-gas mixture introduced into the bottom of the gasifier is 65% steam, 27% H2, and 8% CO.
The raw product gas rises from the top of the upper stage at a superficial velocity of about 1 fps and passes into the inlets of the primary cyclones where most of the entrained particles are captured and returned to the fluidized bed. The finest entrained particles, not captured in the primary cyclones, are carried into the inlets of the secondary cyclones where they are discharged into a common collection vessel.
The secondary fines are subsequently collected in the inlet of a jet ejector from which they are recycled to the riser below the feed injectors. These finest of entrained particles are thus captured by fresh liquid feed droplets and increase in size, being coated with petroleum coke.
The raw product gas, substantially free of entrained particles, flows upwardly from the secondary cyclones into the plenum chamber and is withdrawn from the gasifier overhead. A unit of this capacity would typically have four pairs of cyclones in parallel, all discharging raw product gas into the plenum chamber. Likewise, the secondary cyclone diplegs would discharge into a single common collection vessel connected to the jet ejector inlet.
The composition of the raw product gas is 25% CH4, 13% CO2, 24% H2, 7% CO, 30% unreacted H2O, 0.7% H2S, 0.2% NH3, and trace COS. Heat is recovered from the raw product gas and used to preheat steam and recycle gas, generate steam, and preheat feedstock by conventional methods, as are the gas scrubbing and separations.
As with other gasification processes, the CO2 is available as a concentrated stream suitable for sequestration. Geologic sequestration for enhanced oil recovery described by Melzer is one such possibility.4
Potential application
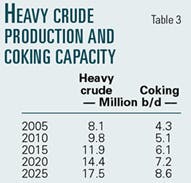
Various projections of worldwide crude production including very heavy crudes show the increasing need for resid conversion capacity over the next 25 years. Table 3 shows the volumes increasing at recent growth rates for heavy crude production and coking capacity in millions of barrels per day. By 2025, coking capacity would increase more than 100% unless other resid conversion processes emerged.
Coking is currently the leading resid conversion process, but in many ways it is analogous to the thermal cracking processes, which were displaced by catalytic cracking in the 1940s. Coking is a nonselective thermal decomposition, which yields less than 60% liquids of low quality. Just as catalytic cracking improved selectivity and yields over thermal cracking, catalytic resid conversion to methane improves both selectivity and yields over coking. Catalytic cracking experience suggests that catalytic resid conversion could capture much of the projected growth in coking capacity as well as conversion capacity for heavy crudes produced specifically for conversion to methane.
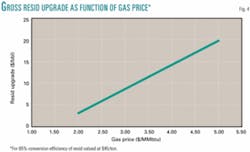
Perhaps the most compelling incentive for commercialization of resid conversion to methane is the remarkable gross upgrade of about $15/bbl for resid conversion to methane, as compared, for example, to about $4/bbl for catalytic cracking of vacuum gas oil. Fig. 4 shows the gross upgrade as a function of gas price for 85% conversion efficiency of resid valued at $45/ton.
Outlook
In the nearer term, the prioritization of feedstocks will always favor liquid feeds with minimum inorganic contamination. Catalytic conversion of resid to methane will be an attractive alternative to coking, and a promising outlet for heavy crudes and bitumens before coal becomes competitive as a feedstock.
In the more distant future, when peak production of petroleum liquids and natural gas finally arrives, the basic technology of catalytic methane synthesis will come full circle and be applied to coal, where it started. The basic chemistry applies equally to any carbonaceous feed, including biomass and municipal waste, providing a high-efficiency conversion of the most foul of combustible material to the cleanest fuel.
In the second of his two-part series in Oil & Gas Journal, Henry Linden lists energy conversion options to provide 100 years of lead time to develop and deploy a global, sustainable, carbon emission-free energy system.5 Catalytic methane synthesis may well be added to the list. F
References
1. Georgetown University: http:// www.ll.georgetown.edu/federal/judicial/dc/opinions/94opinions/ 94-1061a.html.
2. US Energy Information Administration: http://www.eia.doe.gov/pub/ oil_gas/petroleum/data_publications/ petroleum_marketing_monthly.
3. Nahas, Nicholas C., US Patent Application Publication No. US2003/ 0167691 A1, Sept. 11, 2003.
4. Melzer, L.S., OGJ, Jan. 5, 2004, p. 39.
5. Linden, Henry R., OGJ, Jan. 26, 2004, p. 18.
The Author
Nicholas C. Nahas is a retired senior staff advisor from ExxonMobil Research & Engineering Co., where he worked for 33 years in synthetic fuels research, paving materials research, fuels process engineering, and fluid catalytic cracking research and development. He holds a PhD in chemical engineering from the University of Arkansas, where he was named a distinguished alumnus in 1984. He has 23 patents and is the author of several papers and publications. He is currently the director of Petro2020 LLC ([email protected]).