Index correlates asphaltene stability to coker furnace fouling potential
The coking stability index (CSI) test, which determines the relative stability of coker furnace feedstocks, is more accurate than historical analytical methods that evaluate the stability of petroleum fluids.
The correlation of CSI to furnace run lengths provides refiners a better predictive tool to determine the fouling potential of a petroleum fluid.
We measured the CSI for three coking furnace feedstocks and found that the stability of asphaltenes in coker feedstocks has a positive correlation with furnace fouling. We established this link by studying the stability of coker feeds using a solvent titration method with a laser-based solids detection system. We used a near infrared (NIR) laser to measure the relative stability of the asphaltenes in the feed. The laser indicates the onset of asphaltene flocculation during titration of the feedstock with a paraffinic solvent.
Background
The feed to a delayed coking unit is most often the bottoms stream from a crude oil vacuum distillation tower. It therefore contains a high quantity of asphaltenes and resins, both of which are high-molecular-weight species found in crude oils.
Asphaltenes have a long history of actively fouling refinery process equipment.1 This phenomenon is largely due to their presence in crude oil as colloidally dispersed solids.2 As a crude oil is heated, asphaltene stability is disrupted. Asphaltenes experience thermal breakdown, which forms lower-molecular-weight compounds that have reduced solubility in the bulk oil.3
The asphaltene-resin micelle then degrades, which can lead to asphaltene destabilization and agglomeration. Agglomeration of only a few asphaltenes can result in precipitation. Once the agglomerated asphaltenes precipitate from the oil, fouling will ensue.
Asphaltenes are, by definition, a solubility class. They are soluble in hydrocarbon-based liquids with surface tensions greater than 25 dyne/cm (toluene, carbon disulfide, pyridine, or dichloromethane) and insoluble in liquids with surface tensions less than 25 dyne/cm (pentane, heptane, ether).4
Asphaltenes are generally the highest molecular weight and most polar portion of a crude oil. Fig. 1 shows a hypothetical asphaltene structure.5
Resins, as opposed to asphaltenes, have a polar nucleus and one or more nonpolar alkyl branches. The nonpolar branches make the resins more soluble in oil. The polar nucleus, on the other hand, will adsorb on the asphaltenes' surface. This behavior, in essence, makes the resins a natural surfactant for asphaltenes.
Molecular interactions between
asphaltenes and resins fall into several classifications.6 7 Relatively weak polar interactions are one class; another class is hydrogen bonding.
Perhaps the strongest bond, though, is the one due to the interaction of functional groups on the asphaltene and resin. These functional groups contain oxygen, sulfur, and nitrogen atoms, and exist as weak acid and weak base functional groups.
The acid-base attraction between asphaltene and resin represents a third type of bond between these species. Asphaltenes destabilize due to the disruption of these intermolecular bonds, which causes desorption of resins from asphaltenes.
Once exposed, asphaltenes naturally draw together into larger, more polar aggregates. At a certain point, precipitation of the asphaltene aggregate will occur. The end result is deposition and subsequent coking of furnace tubes.
Delayed coker furnace tube fouling lowers the coking unit's operating efficiency, resulting in lost revenues due to reduced production rates and increased maintenance costs.
Experimental methods
The CSI test measures the stability of asphaltenes in furnace feeds via the determination of the onset of asphaltene flocculation point using a solvent titration method.8
Approximately 20 ml of furnace feed mixture is heated and stirred for 25 min. A nonsolvent, such as heptane, is then titrated into the solution and the transmittance of the NIR laser monitored. In the initial stages of titration, the laser's transmittance increases because the added heptane decreases the density of the solution.
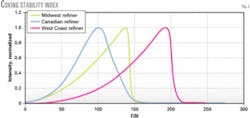
When the asphaltenes begin to flocculate, the laser transmittance will decrease, resulting in an inflection (peak) point (Fig. 2).
The inflection point, known as the CSI, corresponds to the point of asphaltene precipitation and provides a relative measure of asphaltene stability in the test feedstock. A higher CSI means that the coker feed is more stable. One can use this method to evaluate the stability of furnace feeds that have already been subjected to high temperatures.
Results
Table 1 summarizes the traditional analytical measurements used to determine asphaltene stability and coking tendencies, as well as the CSI ranking with the correlating furnace run lengths of three test feedstocks.
We determined the asphaltenes using pentane insolubles and the resins using a Fullers Earth extraction method—modified ASTM D-2007 using chloroform as the extracting solvent. We determined the saturate and aromatic portions using C13 nuclear magnetic resonance.
The colloidal instability index (CII) is the ratio of the saturate and asphaltene fractions to the aromatic and resin fractions.9 A higher CII means that the feedstock is less stable. The asphaltene: resin ratio is also an indicator of feed instability. Feedstocks with an asphaltene-to-resin ratio greater than 0.35 indicates the possibility of stability problems.10 A higher asphaltene:resin ratio means greater instability.
The saturate-to-aromatic ratio predicts instability—a smaller number means higher asphaltene instability in a more paraffinic solution.
Table 1 shows that CSI has the best correlation to actual refinery furnace run lengths as compared to traditional methods of predicting stability.
Fig. 2 shows the CSI in graphical form. The West Coast coker feed has the highest flocculation point, which means that the asphaltenes were more stable and required more of the titrating nonsolvent to start asphaltene flocculation.
The Canadian coker feed was the least stable, and the Midwest coker feed fell between the least and most-stable feedstock. This is a direct correlation to the furnace run lengths experienced in the refineries processing these feeds.
References
1. Asomaning, S., and Watkinson, A.P., "Petroleum Stability and Heteroatom Species Effects in Fouling of Heat Exchangers by Asphaltenes," Heat Transfer Engineering, Vol. 21 (2001), No. 3, pp. 10-16.
2. Tissot, B.P., and Welte, D.H., eds., Petroleum Formation and Occurrence, New York: Springer-Verlag, 1978.
3. Wiehe, I.A., "A phase separation kinetic model for coke formation," Industrial & Engineering Chemistry Research, Vol. 32 (1993), No. 11, pp. 2447-54.
4. Speight, J.G., and Moschopedis, S.E., Chemistry of Asphaltenes, Bunger, J.W., and Li, N.C., eds., Advances in Chemistry Series 195, Washington, DC: American Chemical Society, 1981.
5. Andersen, S.I., and Birdi, K.S., "Aggregation of asphaltenes as determined by calorimetry," Journal of Colloid and Interface Science, Vol. 142 (1991), p. 497
6. Speight, J.G., The Chemistry and Technology of Petroleum, 2nd ed., New York: Marcel Dekker Inc., 1991.
7. Koots, J.A., and Speight, J.G., "Relation of petroleum resins to asphaltenes," Fuel, Vol. 54 (1975), No. 3, pp. 179-84.
8. Stark, J.L., and Asomaning, S., "Crude oil blending effects on asphaltene stability in refinery fouling," Petroleum Science and Technology, Marcel Dekker, Vol. 21 (2003), Issues 3-4, pp. 569-79.
9. Asomaning, S., and Watkinson, A.P., "Deposit formation by asphaltene-rich heavy oil mixtures on heat transfer surfaces," presented to the Understanding Heat Exchanger Fouling and Its Mitigation conference, Castelvecchio Pascoli, Italy, May 11-16, 1997.
10. Al-Atar, E., and Watkinson, A.P., "Effect of Resins on Heat Exchanger Fouling by Asphaltene Containing Oils," presented to the Mitigation of Heat Exchanger Fouling and Its Economic and Environmental Implications conference, Banff, Alta., July 11-16, 1999.
The authors
Thomas J. Falkler ([email protected]) is a senior applications specialist in the fouling control group at Baker Petrolite Corp., Sugar Land, Tex. He has more than 20 years' experience at Baker Petrolite in developing technologies to improve petroleum fluid stability and developing laboratory test methodologies to identify new mitigation and application strategies for refinery fouling. Falkler has authored or coauthored papers and patents on asphaltenic polymers in FCC slurries, oxidation polymerization in naphtha streams, and asphaltene stability in crude oil and furnace feeds.
Joseph L. Stark (Joseph.Stark @BakerPetrolite.com) is manager of the fouling control group and corrosion group at Baker Petrolite Corp., Sugar Land, Tex. He is responsible for product development and technical support in those areas. Stark previously worked as a senior chemist in Baker Petrolite's research and development group. He holds a BA in chemistry from Ohio State University and a PhD in inorganic chemistry from Kansas State University. He is a member of ACS, AIChE, and Phi Lambda Upsilon (honorary chemistry society).