Design guidelines outline solutions for reducing fouling in distillation columns
DISTILLATION—1
When designing mass transfer equipment for fouling service, one must first understand the fouling mechanism, the process in which the fouling occurs, and the process' behavior when the fouling occurs.
Fouling in distillation columns can lead to:
- Greater energy consumption due to heat transfer and efficiency problems.
- Reduced column capacity, which may lead to production losses.
- Increased down time to clean the tower and dispose of fouling materials.
- Potential need for chemical additives.
There are many different opinions of how to design tower internals for fouling services. Because distillation is part art and part science, different designs can work in the field.
Trays and packings can both be used for fouling services. The key to a successful design is the proper application of internals—whether they are trays, packing, or a combination of the two.
This first of two articles presents guidelines for designers to develop—based on their operating experience—practical, serviceable columns for fouling services.
Part 2, next week, will show examples of these design guidelines using case studies in refinery and petrochemical plant operations.
Background
Refiners and petrochemical plant operators are always exploring methods to extend the unit on stream times between maintenance outages. Key equipment attributes that can determine the end-of-run include catalyst life, cyclone erosion, and compressor and tower fouling.
To extend run lengths, plant owners can duplicate the critical equipment that is limiting; for example, parallel pumps, reactors, and reboilers. Although this is a successful method for extending on stream times, it is expensive and, in fact, at times cost-prohibitive. Incorporating design guidelines that increase on stream times of key equipment is a better economic decision for most plant owners.
Currently, refiners aim for 4-year run lengths and ethylene producers aim for longer than 5-year run lengths. These targets present difficulties for distillation columns.
Potential problem areas include refinery vacuum wash-oil beds, ethylene plant quench and saturator towers, and butadiene and other polymer-producing distillation columns. Each of these applications has common traits.
A review of successful and unsuccessful designs can help one identify key design criteria.
Design guidelines developed from successful applications can improve the on stream time in each of these applications.
General guidelines
Distillation column fouling service is a broad term that encompasses many fouling phenomena. The fouling phenomena can occur in the vapor or liquid phase.
If the fouling occurs in the liquid phase, chemical inhibitors can help reduce or eliminate the fouling.
Fouling phenomena include:
- Vaporization of volatile components.
- Polymerization.
- Condensation.
- Sedimentation, precipitation, and crystallization.
- Chemical reaction.
- Corrosion.
Complex fouling problems result if two or more of these phenomena occur at the same time. Ethylene caustic towers, for example, foul due to a chemical reaction in the column; this causes reaction salts to deposit on the tower internals. These factors are almost always mutually reinforcing.
Factors that increase fouling phenomena can include residence time, stagnant zones, sharp transitions, and emulsions. Also, fouling gets worse over time; it is not static.
Fouling mechanisms
In many applications, fouling results if the volatile components vaporize. This fouling occurs in refinery vacuum towers, deasphaltene oil towers, and ethylene quench oil towers.
If the process is placed on recirculationwithout the addition of fresh feed that contains volatile materials, the column can foul when the volatile components are removed.
One ethylene producer, for example, placed the quench oil tower on circulation and increased the viscosity of the bottoms product beyond its pour point. The unit shut down due to fouling and required several days of offline cleaning.
Polymerization occurs when double bonds link to form long-chain molecules. Polymerization products include polyethylene, polypropylene, polystyrene, and polybutadienes.
These products are undesirable in monomer distillation columns and can cause reduced capacity and unit outages.
Condensation is a reaction in which two or more small molecules combine to form large, stable-structure molecules. The formation of coke at high temperatures and long residence times is an extreme form of condensation.
Coke forms when thermal cracking removes hydrogen and light materials from the fluid.
Thermal cracking can occur in gas and liquid phases. The most common situation is liquid-phase cracking when low liquid flow rates increase the residence time in high-temperature operations.
Condensation occurs in ethylene furnace transfer lines to the quench oil tower and in refinery vacuum towers.
Sedimentation occurs when solids accumulate and deposit in low-velocity areas in process equipment.
These types of equipment include heat exchangers, tower distributors, distillation trays, random packing, and structured packing. If a tower's trays are fouled below the feed point the cause might be sedimentation.
Many processes contain suspended solids that can settle out on a mass-transfer surface. These solids include salts, metal oxides, catalyst fines, fermentation products, and coke fines.
Precipitation and crystallization of dissolved salts can occur when the process fluid becomes super-saturated, especially at mass-transfer surfaces.
Ammonia salt deposition due to both water vaporization and direct solid deposition from the gas phase is a common refining problem.
Sometimes the deposits formed do not adhere strongly to the surface. They are self-limiting—the thicker a deposit becomes, the more likely it is that the fluid flow will remove it.
It will therefore attain an asymptotic average value over time.
Velocity strongly affects sedimentation fouling, temperature less so; however, a deposit can bake on a surface and become difficult to remove.
Precipitation and super-saturation are serious types of fouling. Certain salts such as calcium sulfate are less soluble in warm water than cold water. If this type of stream encounters a wall hotter than the saturation temperature of the dissolved salt, the salt will crystallize on the internals' surface.
Crystallization will begin at specific active points (nucleation sites) such as scratches and pits, often after a considerable induction period. The crystals will then spread to cover the entire surface.
Crystallization will continue as long as the surface in contact with the fluid has a temperature above saturation.
The scale is strong, adherent, and can require vigorous mechanical or chemical treatment for cleaning.
These scenarios can cause super-saturation:
- Evaporating solvents.
- Cooling a solution with normal solubility (increases with temperature) below its solubility limit.
- Heating solutions with inverse solubility above their solubility limit. These solutions include CaCO3, CaSO4, Ca3(PO4)2, CaSiO3, Mg(OH)2, MgSiO3, Na2SO4, Li2SO4, and Li2CO3 in water.
- Mixing streams with different compositions.
- Varying pH, which affects the solubility of CO2 in water.
- Deposition of ammonia salts in refining processes.
Additionally, solidification fouling can occur when a stream is cooled below the solidification temperature of a dissolved component, such as the solidification of wax from crude oil.
One can develop guidelines to predict sedimentation of solids from liquids given the fluid and suspended solid properties, flow conditions, and mixing effects.
Petroleum is a mixture of various compounds and the solubility balances among all these components stabilize the petroleum fluid. Changes in that balance can cause precipitation and fouling, such as asphaltene precipitation.
Desired and undesired chemical reactions can occur during distillation. In an ethylene caustic tower, for example, there are competing chemical reactions. Caustic absorbs CO2 (desired) by electrostatic interactions and Van der Waal attraction.
The caustic can also polymerize acetaldehydes (undesired) to first form a yellow oil polymer, and then a red oil polymer via aldol-condensation reactions; these polymers can then create emulsions.
When corrosion occurs, the increased surface roughness can promote fouling from other mechanisms. Bott discusses the various fouling mechanisms.1
Operating conditions
Stagnant zones are where fouling mechanisms can propagate. These mechanisms include vaporization of volatile components, polymerization, condensation, sedimentation, and chemical reactions.
To eliminate stagnant zones, tower designers can specify a small slope for collection devices and flow promoters for distillation trays. Stagnant zones increase residence time in the process and lead to precipitation and buildup of polymer and coke.
Stagnant zones can also promote unwanted chemical reactions due to high residence time and little or no movement of material.
In more severe fouling environments, sharp transitions and corners should be avoided because they are areas where polymer and solids can seed and grow. Polymer and solids can build up in packed tower internals such as feed pipes and trough distributors. Proper design practices can reduce or eliminate the fouling potential of packed tower internals.
If the fouling potential exists in the vapor phase, the tower operator can draw overhead vapor from the tower's side instead of the tower's overhead; this eliminates the additional transition line length and reduces the number of bends and corners.
In hydrocarbon services with water present, such as ethylene quench and saturator towers, emulsions can lead to fouling. If the water's pH varies from neutral ranges, emulsions can occur and create mixtures that carry hydrocarbons.
Trace contaminants like oxygen, nitrogen compounds, sulfur compounds, mercury, and chlorides have a detrimental effect on chemical processes. Designers must be careful with systems that contain contaminants with surfactant properties.
Measures to mitigate fouling
The first step in fouling mitigation is a thorough process review for fouling potential. One should investigate previous applications to develop the best solution for the specific process.
The most suitable mass-transfer equipment for a fouling service may also be the least efficient for mass transfer. Grid packing and shed decks can handle nearly every fouling service, but have low efficiencies compared to sieve trays, and random and structured packings.
For packed towers, the key causes of fouling involve liquid distribution and packing residence time. Longer residence times are less suitable for packings in fouling service. Low pressure drop, smooth surface, low residence-time packings perform best in fouling service.
For fouling environments, the order of preference is: grids, structured packing, and random packing.
In fouling services, distributors increase the residence time and can lead to fouling. In high-fouling services, v-notch or other types of trough distributors are recommended instead of pan-type distributors. The designer should also review the feed piping.
Trays
Industry prefers trays (Fig. 1) in fouling services due to the long history of success trays have had in fouling applications.
null
The first continuous distillation column with bubble cap trays was built in 1813; structured packing was first developed in 1964. The fouling application database for trays, therefore, is much larger.
The best trays to use in fouling services are sieve trays and dual-flow trays. Moveable-valve trays are more prone to fouling because fouling can seed and propagate on the valves.
In fouling services, downcomer design requires attention to detail. A major problem with downcomers is dead spots.
These often occur near the tower wall, opposite the downcomer outlet, near outlet weirs, and at the ends of the downcomer.
Modified downcomers that decrease the downcomer volume can help reduce dead spots by keeping more of the downcomer volume full of agitated liquid.
In quick revamps of existing equipment an alternative is to install metal or ceramic shapes to modify the downcomer volume. Designers must consider the influence of weir heights and avoid inlet weirs when possible.
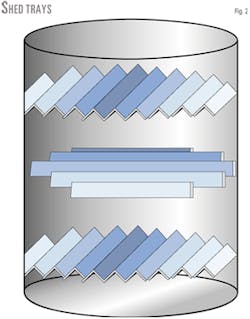
Shed decks (Fig. 2) are essentially 2-10 in. angle iron beams that are placed in rows across the column, typically on a 24-in. tray spacing. They can be set in overlapping rows or rotated 90° from tray to tray. The open area on the tray is typically 50%.
Shed decks have the advantage of almost no fouling potential because there are no stagnant zones and they have a low residence time.
The tray's efficiency unfortunately almost matches the fouling potential, especially for wide shed decks. Shed decks work well if the application is essentially for heat-transfer purposes.
null
These trays have low fouling potential and efficiency.
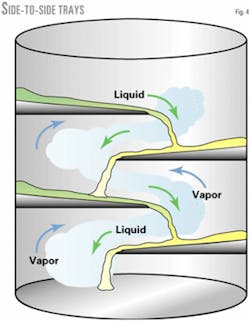
Side-to-side trays (Fig. 4) allow the liquid to splash from side to side; the decks can be sloped. These trays also have low fouling potential and efficiency.
Dual-flow trays are preferred for heavy fouling services. Dual-flow trays have no downcomers where fouling products usually accumulate or where polymer and solids can seed and propagate.
Dual-flow trays have enough open area on the tray decks to eliminate stagnation and promote back mixing.
A disadvantage with dual-flow trays is that they have a limited turndown potential.
Vapor and liquid flow up and down the column through holes on the tray deck. This helps eliminate vapor-phase fouling because the tray's underside is washed.
Continuous agitation of liquid on the top of the trays combined with continuous underside wetting and washing action makes this tray suitable for fouling services.
The disadvantage of dual-flow trays is maldistribution in larger-diameter towers. During a typical storm, a column's top can move as much as 6 in. This movement can cause a hydrologic flow instability that can propagate down the column. Improper feed, reflux, or vapor distribution can also create maldistribution problems.
Two types of dual-flow trays are available—standard deck and rippled deck. The standard deck has a flat plate, and the rippled deck has sinusoidal waves.
Spray nozzles vs. distributors
There is much disagreement in the industry on the advantages and disadvantages of spray nozzles in fouling services. Some advocate only spray nozzles with no packing or shed decks. Others advocate only distributors with packing.
The best trough distributor has better distribution than the best spray distributor. In fractionation services, the separation requirements may mandate the use of a trough distributor.
In solids services, such as FCC slurry zones, specially designed trough distributors have proven their performance.
Spray distributors resist plugging if the nozzles on a spray distributor have a large enough minimum flow passage to protect against plugging.
Spray distributors have performed well in extremely severe services including refinery vacuum wash zones and delayed coker main fractionators.
Construction materials, finishes
The right type of material or, more importantly, surface finish can reduce fouling.
Smooth surfaces shed deposits more easily than rough surfaces. A corroded surface is more prone to other fouling mechanisms.
One can select a suitable material for fouling services using these guidelines:2
- Standard austenitic stainless steels, such as 316 (18Cr, 10Ni, 3Mo), provide resistance to acids and reasonable resistance to pitting corrosion. Type 304L (18Cr, 10Ni) stainless steel has a good resistance to nitric acid. Austenitic stainless steels have relatively low strength, poor antierosion and abrasion properties, and do not resist stress-corrosion cracking.
- Super-austenitic stainless steels with relatively high nickel content (approximately 20Cr, 29-34Ni), sometimes referred to as Alloy 20, are more costly than standard austenitic steels but provide excellent resistance to acids and some acid chlorides.
- Duplex stainless steels offer high strength and resistance to abrasion, erosion, and stress-corrosion cracking.
- Nickel-based alloys, such as Hastelloy, have outstanding corrosion resistance to acids, mixed acids, and acids at high temperatures. The main disadvantage is high cost.
Additives
An additive can resist fouling by:
- Chemically reacting with the fouling species to modify its fouling potential.
- Changing the physical interaction between the foulant and equipment surface.
- Modifying surface deposits so they can be removed by fluid flow.
Pretreating the metal with passivating solutions can also lower fouling potential.
References
1. Bott, T.R., "Fouling of Heat Exchangers," Elsevier Science, Amsterdam, 1995.
2. Muller-Steinhagen, H., ed., "Heat Exchanger Fouling—Mitigation and Cleaning Technologies," IchemE, Publico Publications, Essen, Germany, 2000.
Based on a presentation to the American Institute of Chemical Engineers 2004 Spring National Meeting, Apr. 25-29, 2004, New Orleans.
The authors
Karl Kolmetz ([email protected]) is an assistant manager of process technology for Sulzer Chemtech Pte. Ltd., Singapore. His responsibilities include technical support for process equipment design and installation for Asia. He previously worked for the Westlake-Titan Group, Ray- theon Badger Construction Co., and Charter-Phibro Refining Inc., Houston. Kolmetz holds a BS in chemical engineering from the University of Houston.
Wai Kiong Ng ([email protected]) is senior engineer for Sulzer Chem- tech Pte. Ltd., Singapore. Ng holds a masters in chemical engineering from Technical University of Clausthal, Germany. He is a member of Verein Deutscher Ingenieure (German engineers' association).
Peter W. Faessler ([email protected]) is the Asia-Pacific general manager for applied process technology for Sulzer Chemtech Pte. Ltd., Singapore. He joined Sulzer in 1982 with assignments in Switzerland, South America, and Asia. Faessler has a degree in chemistry and chemical engineering from Basle Engineering College, Muttenz, Switzerland.
Andrew Sloley ([email protected]) is a principal engineer for VECO USA Inc., Bellingham, Wash. His responsibilities include training, process consulting, design, and design supervision. He previously worked as a troubleshooter and consultant for Glitsch, Inc. and as a process engineer in technology development and application for Exxon Chemical Co. Sloley holds a BS in chemical engineering from the University of Tulsa and is a registered professional engineer in Texas.
Timothy M. Zygula ([email protected]) is a senior process engineer for Nova Chemicals Corp., Pasadena, Tex. Previously, he was a senior process engineer with the Westlake Group, Lake Charles, La., and a process engineer with Glitsch, Inc. Zygula holds a BS in chemical engineering from the University of South Florida and an MS in chemical engineering from McNeese State University, Lake Charles. he is a member of AICHE and is a licensed professional engineer in Louisiana.