Pressurized reboiler reduces VOC emissions in glycol dehy systems
A pressurized glycol reboiler, first used at a Shell Oil Co. South Louisiana plant, is effective in controlling volatile organic compound (VOC) emissions from glycol gas-dehydration systems.
The reboiler operates at pressures that allow the overhead vapors that contain VOCs and stripping gas to flow directly to compressor suction, a fuel system, or to a flare. These reboilers effectively control benzene, toluene, ethylbenzene, and xylene (BTEX) emissions and economically recover all the hydrocarbon vapors with minimal incremental capital cost and no required emissions monitoring.
BTEX and other VOC emissions are an environmental challenge for the natural gas industry. VOCs are considered carcinogenic and occur in most natural gas streams.
The most common gas-dehydration systems use triethylene glycol (TEG) to adsorb water from natural gas. Unfortunately, TEG also adsorbs VOCs that vaporize with the water in the reboiler.
Due to the number of glycol dehydration systems in use (more than 40,000 in the US) and the estimated amounts of VOCs they absorb, federal and state organizations and many foreign governments require monitoring and control of VOC emissions.
Industry has expanded much effort in developing predictive methods (OGJ, Oct. 16, 1995, p. 60) and assuring measurement standards to meet Clean Air Act requirements.
VOC emissions
VOC control methods fall into two groups. In the first group, VOCs are returned to the gas stream, flared, or used as fuel. These are essentially zero-emission methods.
In the second group, VOCs are recovered, then used or disposed of. Because the volume of VOC emissions is significant from an environmental standpoint, but the emissions have little sales value, these methods are generally used where there are other reasons to select them. For example, Drizo systems provide high glycol concentrations.
VOC emission control methods currently used with glycol dehydration systems include simpler (Group I) and more complex (Group II) methods.
Group I includes:
- Vapor recovery units (VRUs). Overhead vapors from the glycol reboiler are cooled or mixed with other gases and compressed to feed the sales gas compressor.
- Ejectors. A subset of VRUs, ejectors use high-pressure gas to compress the vapors, which are then used as fuel or flow into another compressor.
- Pressurized reboiler. This eliminates the need for a VRU for still overhead vapors; however, the maximum practical pressure is about 70 psig.
- Group II includes:1
- Condensers that use air, water, etc., as the coolant. Condensed liquids are separated and noncondensable components are burned as fuel or flared. The hydrocarbon liquid combines with the oil stream.
- Drizo. This system uses an azeotropic distillation method to produce a high-purity glycol (99.99%). It can use the VOCs as a solvent. The Drizo system contains an overhead condenser, solvent heater, glycol pump, and solvent-water separator.
- Membrane Technology & Research Inc.'s PerVap system. The process includes condensation using air cooling, separation using a three-phase separator, and a membrane system to clean the waste water stream. The system strips essentially all the dissolved hydrocarbons from the aqueous stream and produces relatively clean water.
Many of these control systems require frequent sampling to ensure that the required percentage of VOC vapors is not released to the atmosphere. The VRU does not have this problem but is operationally more complex due to the low flow rates and low suction pressures.
Because VOCs are water soluble, some units such as PerVap remove VOCs from condensed water so that operators can dispose of it as "nonhazardous."
History
Shell has used a pressurized reboiler in two applications that have resulted in zero emissions. In these systems, the reboilers operate at field separator pressures (50-70 psig) with the still overhead vapors routed to the field gas compressor's suction. This process eliminates VOC emissions in the gas phase and reduces them substantially in condensed water.
The entire overhead stream can mix with the main low-pressure gas production. Because there are no emissions, one does not need to calculate the quantities of VOCs or test periodically.
Louisiana and Texas have accepted this design as emission free and, therefore, no sampling is required.
In 1992, Shell's Weeks Island plant near New Iberia, La., needed more dehydration capacity. A study determined the best method for controlling VOCs was a new system developed by Hicks & Associates that used a pressurized reboiler.
The patented design (US Patent 6375806) was assigned to Allen Process Systems.
The Weeks Island unit's reboiler operated at 50-60 psig to allow all the vapors from the still overheads to enter the suction of the existing compressor stations. The unit was installed and operated successfully for 5 years, at which time it was no longer required.
In 2001, Shell commissioned a similar study for its McAllen, Tex., field. Shell wanted to expand the McAllen unit capacity to 250+ MMscfd from about 200 MMscfd.
Because McAllen had a central compressor station with suction pressures of 50-60 psig, the study recommended a pressurized reconcentrator. The pressurized unit began operation in the first quarter 2003 and is performing as expected (Fig. 1).
Final McAllen design, selection
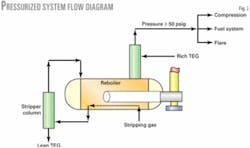
Fig. 2 shows the flow scheme for the McAllen glycol system with the pressurized reboiler. The flow scheme is similar to atmospheric reboiler systems—the pressure operation presents many alternatives for VOC disposition.
Shell specified these elements for the McAllen design:
- System operating pressure and outlet-gas water content.
- Two complete trains, both contacting and reconcentration, each designed for 50% of the total capacity.
- Kimray pumps instead of electric motor driven, positive-displacement pumps.
- A pressurized system with a reboiler operating pressure of 60 psig to allow all VOCs to enter the first-stage suction of the field-gas compressor.
With these items defined, the sizing process determined lean TEG concentration (stripping gas rate and number of contacts), the number of gas-glycol contacts (height of packing), and the lean-glycol circulation rate. The design process worked with all of these interrelated elements to arrive at the optimum solution.
We used a glycol dehydration program (PC Glycol) for most of the process and mechanical calculations. The program includes the most recent experimental data.2 3 Shell and others have used this program for more than 15 years and found it to be a accurate predictor of actual process performance of glycol units.4 5 The program's current version was improved to handle cases with pressurized reboilers, which can also apply to lower pressures, such as high-altitude cases.
Stripping gas design
The stripping gas system was specified for reboiler operating conditions of 60 psig and 390° F. The stripper design is important because the countercurrent stripping column performs most of the water-removal duty in pressurized reboilers.
Although the glycol boiling point is 550° F., the TEG degradation temperature is only 404° F. The maximum normal operating temperature (400° F.) at atmospheric pressure will result in only 98.9% lean glycol. Any pressure increase reduces this value substantially. For example, a reboiler pressure of 60 psig results in only 93.5% lean TEG.
An important design consideration is the vertical distance between the reboiler and glycol surge tank. This distance must include the stripping column and account for the pressure drop in the glycol-glycol heat exchangers (usually 1-2 ft). The glycol level should not back up into the stripper; otherwise the column will flood, lower the efficiency, and cause probable glycol losses.
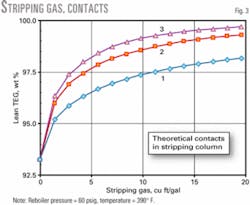
More packing increases the theoretical contacts in the stripper; this decreases the quantity of stripping gas required for a particular glycol concentration.
Fig. 3 shows that the lean TEG improvement between one and two contacts (theoretical plates) is substantial, while that from two to three is not as great but still could provide substantial reductions in stripping gas rates for specific lean TEG concentrations.
Because the stripping column is small (12 in.), random packing is used. The height equivalent to a theoretical plate (HETP) for random packing in this service will be 2-3 ft.
The stripping column has about 6 ft of random packing because the reboiler's physical layout over the surge tank will easily allow for this amount. We selected 1-in. stainless steel Pall rings to provide adequate capacity for future possible increases.
With 2-3 ft of packing/theoretical contact, the column will provide two to three theoretical contacts, with a value of two used for further calculations.
Given two contacts in the stripper, we performed various calculations for glycol circulation rate, reboiler heat duty, and contactor packing height. The calculations resulted in a normal operating stripping gas rate of 10 cu ft/gal and a maximum design rate of 20 cu ft/gal.
Fig. 3 includes the impact of a temperature decrease in the stripping column due to latent heat and heat of solution of water stripped from the glycol. For units with higher operating pressures and lower circulation rates, in terms of gal/lb of water removed, this factor can be important.
The Shell units included connections to allow glycol recirculation from the stripper's bottom back to the reboiler. This provision allows one to increase the glycol concentration by effectively raising the stripper column temperature due to more heat energy into the stripper column from recycled glycol. This allows the dehydration system to operate satisfactorily with higher gas temperatures or higher reboiler pressures.
Contactor structured packing
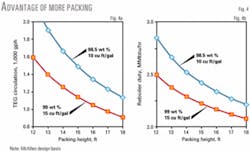
Most structured packings have an HETP of 4.5-5.5 ft. Although newer packings provide HETPs of 3.5-4.0 ft, we used the higher value of 5.5 ft for the McAllen calculations to ensure adequate contact. Because these were new towers, the additional cost of the conservative design was low.
Fig. 4 shows the circulation rate and reboiler heat duty reductions as the packing height increases. This shows that more stripping gas can reduce the circulation rate and required reboiler duty better than increasing the packing height. We selected 15 ft of packing because:
- The operator can increase the stripping gas rate to 20 cu ft/gal if needed.
- The design gas temperature of 115° F. will occur only during certain months.
- The HETP of 5.5 ft is probably conservative for the contactor, as is the number of stripping contacts.
The 5.5-ft HETP with 15 ft of packing provides 2.7 theoretical contacts.
Lean glycol circulation, heat duty
With all the other variables selected, we determined the final glycol circulation rate.
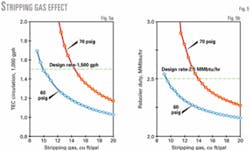
Fig. 5 shows the circulation rate and reboiler heat duties for two reboiler pressures—the 60-psig design case and a "safety" pressure of 70 psig in case the compression suction pressure increases.
These curves show that the minimum glycol circulation rate is about 1,200 gph. Because this only reduces the heat duty to 2.25 MMbtu/hr from about 2.5 MMbtu/hr, we selected a design rate of 1,500 gph to allow for future unexpected conditions. The reboiler design was set at 2.5 MMbtu/hr.
Operations
The units were started up in March 2003 with only minor problems.
The computer program predicted unit performance so well that we developed enhanced versions of the program, which allow operators to fine tune the glycol circulation and stripping gas rates for changes in gas conditions (rate, pressure, and temperature) and reboiler operating pressure.
Advantages
The pressurized glycol reconcentration system offers advantages that include:
·All vapors (VOCs, H2S, CO2) released from the glycol are at a substantial pressure, which allows the use of simple handling methods without discharge to the atmosphere. Typical handling methods are:
- Mixing the overheads with fuel gas or other gas users.
- Recompression with compressors in other service instead of dedicated compression.
- Dedicated compression. With this handling method, reboiler pressure operation allows for much better control due to the higher suction pressures.
·Because the previously mentioned methods can handle vapors from the still overheads, one can eliminate the equipment specifically designed to recover these vapors.
This equipment typically includes condensers, separators, pumps, and a means for burning or incinerating the noncondensable vapors.
For Weeks Island, the entire still column overhead was routed to the compression suction scrubbers with no intervening components like a condenser or separator.
·The larger-than-normal amount of stripping gas used in the stripper results in less VOCs in the condensed water vapor from the overheads. This is because the vapor-liquid equilibrium is shifted such that condensation of VOCs is minimized. The disposal water will therefore contain significantly less undesirable components.
Similarly, for those systems in which the overheads are mixed with other gases, the volume ratio of overhead to mixing gas (such as gases for fuel and compressor suction) will further reduce the VOCs in the condensed water.
·No environmental testing for atmospheric discharge of VOCs is required because no VOCs will be released, as is often the case with conventional technology. Furthermore, discharge of VOCs from the reconcentration system at considerably higher-than-normal pressures facilitates mixing and extreme dilution of VOCs in plant fuel or in fuel sold and transmitted to remote users.
Acknowledgment
The authors thank Shell Exploration & Production Co. and Allen Process Systems for allowing publication of this work.
References
- "Evaluation of BTEX Emission Mitigation Systems for Glycol Dehydration Units," GRI-93/0147, Gas Technology Institute, Des Plaines, Ill., June 1993.
- Parrish, W.R., Won, K.W., and Baltatu, M.E., "Phase Behavior of the Triethylene Glycol-Water System and Dehydration/Regeneration Design for Extremely Low Dew Point Requirements," presented to the 65th GPA Annual Convention, San Antonio, Mar. 10-12, 1986.
- Aogagi, K., Song, K.Y., Sloan, E.D., Dharmawardhana, P.B., and Kobayashi, R., "Improved Measurement and Correlation of the Methane Gas in Equilibrium with Hydrate," presentation to the 58th Annual GPA Convention, Denver, Mar. 19-21, 1979.
- Hicks, R., and Senules, E.A., "New gas-water-TEG equilibria," Hydrocarbon Processing, April 1991.
- Youn, K.C., and Hicks, R., "Improved Program for Natural Gas Dehydration with TEG," presented at the 65th Annual GPA Convention, San Antonio, Mar. 12-13, 1991.
The authors
Ralph Hicks is president of PetroDesigns, Marrero, La. He worked for Shell Oil Co. from 1968 to 1974 as a facilities engineer and Natco Group from 1974 to 1981 as an engineering manager. From 1981 to 2000, Hicks managed his own companies that were all involved in the design and fabrication of oil and gas processing facilities. He holds a BS (1965) in electrical engineering from Louisiana Tech University, Ruston.
Dale Gallaher is a senior staff engineer at PetroDesigns, Marrero, La. He previously worked for Shell Oil Co. from 1974 to 1998 as a facilities project engineer on facility projects in the Gulf of Mexico and overseas. He holds a BS (1962) in physics, an MS (1968) in electrical engineering from Oregon State University, Corvallis, and an MS (1989) in computer engineering from Tulane University.
Richard Craig is a staff facilities engineer with Shell Exploration & Production Co., Houston, supporting their South Texas gas production assets. He has worked in support of exploration and production surface facilities since May 1998. From 1985 to 1998 Craig worked for Shell Pipeline Corp. in various field and head-office engineering assignments. He holds a BS (1980) in electrical engineering from the University of Houston.