Tesoro refinery improves operation efficiency in amine treating unit
The Tesoro refinery, Kapolei, Ha., improved operation of its hydrogen plant amine unit by switching to a specialty amine. The methyldiethanolamine (MDEA)-based solvent reduced system corrosion, and lowered additive and solvent makeup rates.
The plant significantly lowered iron losses and corrosion since converting to the specialty amine in August 1999. Because the unit experienced much longer operating times without the need for solvent makeup—38 months of operation—overall operational costs were significantly reduced.
Tesoro refinery
Tesoro's hydrogen plant amine unit has used several different amine solvents in the past. Similar to other deep CO2-removal amine systems, the plant experienced corrosion problems and high solvent losses.
Inhibited monoethanolamine (MEA) was the first solvent used in this plant. Due to the energy savings available with MDEA-based solvents, and the disposal and health concerns associated with inhibited MEA, the plant owner wanted to convert to an MDEA-based solvent.
The plant started using a first generation MDEA-based amine in 1987 and later converted to a second-generation MDEA product in 1991.
In August 1999, the unit started using GAS/SPEC's CS-2020 solvent. Since the conversion, the plant has virtually eliminated corrosion from the system. This has resulted in significant cost savings and increased hydrogen production by eliminating unplanned shutdowns due to equipment failure and by shortening the time and manpower required for shutdowns and inspections.
The operator also eliminated the need for routine additions of promoters or additives used with the first and second generation MDEA-based solvents to facilitate the CO2 removal. These promoters required the plant operator to add the makeup promoter separately of the general solvent.
After converting to the specialty amine, overall solvent makeup rates were also dramatically reduced.
Other benefits include higher capacity and CO2-removal efficiency and lower methanator delta temperatures.
Corrosion
The hydrogen amine plant historically had high corrosion and significant iron carbonate fouling. Iron levels in the circulating solution were 50-590 ppm before the solvent conversion in 1999.
Although iron levels in solution do not necessarily correlate to high corrosion rates, equipment metal losses and iron carbonate formation indicated that corrosion was occurring.
Tesoro significantly reduced the corrosion and fouling at yearend 1997 when the carbon steel cross exchanger bundle was replaced with a stainless steel one. This is the standard metallurgical preference for this service.
Tesoro also began to aggressively filter the solution with carbon to minimize degradation products that could contribute to corrosion.
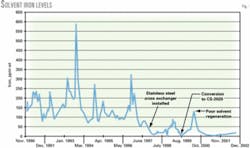
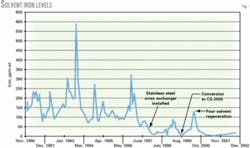
Fig. 1 shows the iron levels during 1990-2002 for the system. The iron level has been consistently low since the solvent conversion. Only once did the iron level exceed 100 ppm; this coincides with an operational upset in the regenerator that caused high lean loadings.
Average iron in solution for the second-generation solvent was 91 ppm vs. 21 ppm for the specialty amine.
Table 1 shows historical iron levels and average solution chemistry data for the specialty amine and previous solvent.
INEOS Oxide Ltd. conducted accelerated autoclave corrosion testing that exposed different solvents to CO2 at pressures and temperatures representative of CO2-only deep removal processes. The corrosion rates for the first-generation, second-generation, and specialty solvent were 58 mils/year (mpy), 5 mpy, and 1 mpy, respectively.
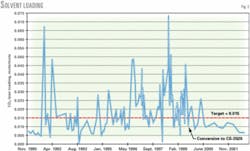
Fig. 2 shows the CO2 lean loadings since the 1999 conversion. Loadings were greater than the recommended guidelines a couple of times since the conversion.
The main cause of the deviations was a plug in the inlet line to the steam reboiler that affected amine regeneration. The plug consisted of pieces of iron carbonate that probably got wedged into the line due to the absence of a suction guard plate when the packing was last dumped before the conversion.
The new solvent also reduced the iron carbonate fouling that the plant previously experienced. With the previous solvents, the level control valve on the rich amine line to the stripper had to be stroked weekly to keep it functioning properly. Scale would otherwise build up on the control valve and impair operations, eventually requiring a shutdown for maintenance. Since the conversion, the operator has observed no iron carbonate on this valve.
During shutdowns, the operator also found significant iron carbonate deposits in the cross exchanger (rich side) and throughout the stripper.
Fig. 3 shows iron carbonate fouling in the stripper during the last shutdown (in 1998) before the conversion to the specialty amine.
The iron carbonate fouling was so severe that it had fused the packing together; jackhammers were needed to remove it from the regenerator.
Fig. 4 shows the stripper packing a year after the conversion. The packing does not show any sign of fouling or damage. This is an improvement compared to previous shutdowns.
The plant has maintained the same CO2 treating ability. During previous shutdowns, inspectors opened all major vessels. Due to the lack of process problems and the low iron levels in solution, only the regenerator was opened for inspection during the latest shutdown. The regenerator was opened mainly to remove a plug in the suction line to the steam reboiler.
Due to a history of corrosion, the hydrogen plant corrosion-monitoring program consisted of ultrasonic-thickness measuring by Tesoro, corrosion probe and coupon testing by Nalco Co., and solution corrosivity analysis by INEOS Oxide Ltd.
Because corrosion was reduced significantly in the process (Fig. 5) and due to the low iron numbers in solution, corrosion probe and coupon testing was discontinued near the end of 2001.
Fig. 5 shows the quick drop off in corrosion rate after the solvent changeout. The corrosion rate was 0 mpy for so long that Tesoro stopped testing.
Solvent performance
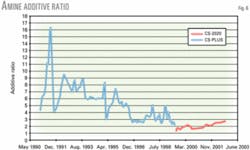
The new solvent is more stable as shown by the fact that routine separate additions of a promoter to the system have been eliminated since the conversion.
Table 2 shows the results of accelerated autoclave solvent degradation testing.
Previous plant operations required periodic make-ups with concentrated solvent and a standard solvent formulation.
After 3 years of plant operations with the specialty amine, overall operations are more consistent as proven by the more stable chemistry and operating parameters, such as lean loadings (Fig. 2) and additive ratio (Fig. 6).
After the conversion, solvent use rates were about 4 lb/MMcf of gas processed. With the second-generation solvent, Tesoro was using about 14 lb/MMcf of gas. This reduction represents a significant chemical cost savings.
Table 3 shows Tesoro's operations with the three different types of solvents.
Based on a presentation to the 53rd annual Laurance Reid Gas Conditioning Conference, Feb. 23-26, 2003, Norman, Okla.
The authors
Jon Taketa is a process engineer at Tesoro Petroleum Corp., Kapolei, Ha. He holds a BS (1993) in chemical engineering from the University of California, Berkeley.
Joe Drogowski is a training superintendent at Tesoro Petroleum Corp., Kapolei. He has 23 years' experience in refinery operations and has also served as operations process superintendent, process shift supervisor, and process operator for Tesoro Petroleum Corp.
Norman Edelman is an operations/maintenance superintendent at Tesoro Petroleum Corp., Kapolei.
Randy Kuroda is a senior gas treating engineer for the GAS/SPEC Technology Group of INEOS Oxide, Campbell, Calif. He holds a BS in chemical engineering from the University of California, Berkeley.