Correlation calculates solubility, Henry's Law constant for olefins, diolefins in water
A new correlation calculates water solubility and Henry's law constants for a wide variety of olefins and diolefins in water. The new correlation, based on boiling point temperature, provides reliable solubility values down to low chemical concentrations in water.
Data from the correlation and experimental data agree favorably. The results, provided in an easy-to-use tabular format, are especially applicable for rapid engineering use for health, safety, and environmental studies.
Water solubility
Understanding the solubility of olefin and diolefin compounds in water will have more importance in the future due to heath, safety, and environmental issues.
For example, the safety-related lower explosion limit for 1-pentene in air is 1.5 vol %.1
A concentration of 0.00001 mole fraction of 1-pentene in water provides about 22.2 vol % of 1-pentene in air at the air-water interface, which exceeds the lower explosion limit of 1.5 vol %.
For an example of the environmental impact of olefins and diolefins, consider the impact of a spill of 1-pentene in contact with water.
The water will become saturated with 1-pentene. At saturation, the solubility of 1-pentene in water is about 0.00003802 mole fraction.2 This concentration at saturation provides about 84.4 vol % of 1-pentene in air at the air-water interface, which exceeds the lower explosion limit of 1.5 vol %.
This brief example using 1-pentene indicates that low concentrations of olefin compounds in water can provide concentrations in air at the air-water interface that exceed the lower explosion limit for flammability.
Correlation for water solubility
In earlier works, Yaws and coworkers (OGJ, Apr. 8, 1991, p. 79; Sept. 16, 1991, p. 86; Aug. 29, 1994, p. 80) correlated water solubility for hydrocarbons and other chemicals as a function of the compound's boiling point.1 3
The new correlation also uses the boiling point method for correlating water solubility of olefin compounds:
log10(S) = A + B 3 TB + C 3 TB2 + D 3 TB3
where:
S = solubility in water at 25° C, ppm (wt).
TB = boiling point temperature of compound, K.
A = 217.0300 for olefins, 216.5610 for diolefins.
B = 177.811E-03.
C = 2500.907E-06
D = 411.124E-09
The range for boiling point temperature is 310-560 K.
We determined the coefficients from regressing available data. We conducted a literature search to identify data source publications.1-11
Compilations of Howard and Meylan,8 Verschueren,11 Yalkowsky,2 and Yaws,1 3 were used extensively. We surveyed the data sources to create a database of experimental information, which also served as a basis to check the accuracy of the correlation.
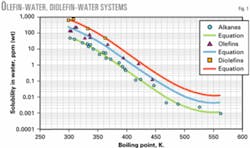
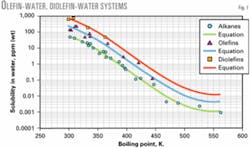
Fig. 1 shows a graph of water solubility vs. boiling point temperature for olefins and diolefins. More data were available for alkanes than olefin compounds.
Also, the graphs show that data for olefins and diolefins appear to be slightly above and approximately parallel to the more extensive data for alkanes. This suggests that the solubility of olefins and diolefins is higher and about parallel to the water solubility of alkanes.
Water solubility, Henry's Law constant
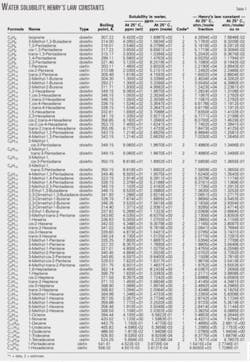
Table 1 shows results for water solubility and Henry's Law constant.
The results for Henry's Law constant are based on water solubility and vapor pressure at ambient conditions using the appropriate thermodynamic relationships. The presented values are applicable for olefins and diolefins.
The tabulation is arranged by carbon number, which makes it easy to quickly locate data using the chemical formula.
Example calculations
A chemical spill of 1-hexene occurs into a body of water at ambient conditions (25° C., 1 atm.). Estimate the concentration of 1-hexene in water at saturation.
Substitution of the correlation constants and boiling point temperature for 1-hexene into the correlation yields: log10(S) =.2.17.0300 + 1.77811E-01.3.(336.63) 2 5.00907 E-04 3 (336.33)2 + 4.11124E-07 3 (336.33)3
S = 55.84 ppm (wt)
A chemical spill of 1-pentene occurs in a body of water. The concentration (wi) of 1-pentene in the liquid at the surface of water is 10 ppm (mole). Estimate the concentration of 1-pentene in the air at the surface of water.
From thermodynamics at low pressure, the vapor concentration is: yi = (Hi/Pt) wi
where:
yi = Vapor concentration of component i, mole %.
Hi = Henry's Law constant.
Pt = Total pressure, atm.
wi = Liquid concentration of component i, mole %.
Substitution of Henry's law constant from the table, total pressure of 1 atm, and liquid concentration into the above equation provides:
yi = 22190 3 0.00001
yi = 22.19 mole %
Acknowledgment
The Texas Hazardous Waste Research Center at Lamar University, Beaumont, Tex., provided partial support for this work.
References
1. Yaws, C.L., "Chemical properties handbook," New York: McGraw-Hill Cos., 1999.
2. Yalkowsky, S.H., "Aquasol database," University of Arizona, Tucson, 1990-2002.
3. Yaws, C. L., "Yaws' handbook of thermodynamic and physical properties," Norwich, NY: Knovel Corp., 2002.
4. "CRC handbook of chemistry and physics," 75th-82th eds., Boca Raton, Fla.: CRC Press Inc., 1994-2002.
5. Donald, M., Shui, W.-Y., and Ma, K.-C., "Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals," Vol. 4, New York: Lewis Publishers, 1995.
6. "Handbook of environmental data on organic chemicals," 4th ed., New York: John Wiley & Sons Inc., 2001.
7. Hine, J., and Mukherjee, P.K., J. Org. Chem., Vol. 40 (1975), p. 292.
8. Howard, P.H., and Meylan, W.M., eds., "Handbook of physical properties of organic chemicals," Boca Raton, Fla.: CRC Press, 1997.
9. Mackay, D., Shiu, W.-Y., and Ma, K.-C., "Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals," Vols. 1-5, New York: Lewis Publishers Inc., 1992-97.
10. Suzuki, T., Journal of Computer-Aided Molecular Design, Vol. 5 (1991), p. 149.
11. Verschueren, K., "Handbook of environmental data on organic chemicals," 3rd and 4th eds., New York: Van Nostrand Reinhold Co., 1996, 2001.
The authors
Carl L. Yaws is a professor of chemical engineering at Lamar University, Beaumont, Tex. His research interests include technology development, thermodynamic and transport property data, environmental engineering, and process simulation. Yaws holds BS in chemical engineering from Texas A&I University, Kingsville, and an MS and PhD in chemical engineering from the University of Houston. He is a registered professional engineer in Texas.
Harman Singh is obtaining an MS in chemical engineering at Lamar University. His research interests include thermodynamics, environmental engineering, and process simulation. Singh holds a BS in chemical engineering from Punjab Technical University, Jalandhar, India.
Praveen Bajaj is obtaining an MS in chemical engineering at Lamar University. His research interests include thermodynamics, environmental engineering, and process simulation. Bajaj holds a BS in chemical engineering from Siddaganga Institute of Technology, Tumkur, India.
Ralph W. Pike is Paul M. Horton Professor of Chemical Engineering and the director of the Minerals Processing Institute at Louisiana State University, Baton Rouge. His research interests include process optimization, fluid dynamics, reactor design, ecological systems, and pollution prevention. Pike holds a BS and PhD from Georgia Institute of Technology, Atlanta. He is a registered professional engineer in Louisiana and Texas.