Japan refiner improves FCC operations using catalyst separation technology
In September 1999, Kyokuto Petroleum Industries (KPI) commissioned a MagnaCat catalyst-separation unit in its resid fluid catalytic cracker (RFCC) at its Chiba, Japan, refinery.
KPI observed these operating benefits, in order of economic importance:
- Yield improvements at a constant catalyst-makeup rate.
- Higher feed vacuum resid content due to improved coke selectivity.
- Reduced catalyst makeup rate at constant equilibrium activity.
The economic benefit attributable to MagnaCat is as much as $0.47/bbl of feed when the unit operates in a maximum gasoline mode against a catalyst cooler limitation.
These results confirm studies1-6 showing that removing the oldest FCC catalyst particles via magnetic separation reduces coke and gas production from dehydrogenation and increases selective catalytic cracking.
The catalytic advantages provided by MagnaCat allow processing higher rates of equivalent quality feed or poorer quality feeds at equivalent rates.
Basic principles
Refiners add fresh catalyst to FCC units on a near-continuous basis to offset deactivation and physical losses. In most cases, the catalyst-addition rate required to maintain activity is higher than the physical losses, and equilibrium catalyst (e-cat) must be withdrawn and discarded from the regenerator.
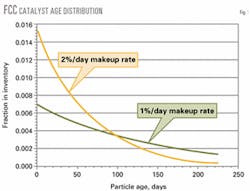
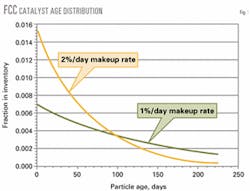
Fig. 1 shows a typical age distribution for two different daily catalyst makeup rates.
Particles range from high-activity, recently added fresh catalyst to catalyst particles that have been in the inventory many months and are catalytically dead due to accumulation of metals and long-term exposure to high temperatures and steam in the regenerator.
Because the inventory is well-mixed, withdrawing e-cat for metals control results in the removal of fresh active particles with the old, deactivated particles.
As FCC catalyst particles age, they accumulate iron, nickel, and vanadium; the metal oxide forms of these metals are magnetic and are attracted to high-field-strength magnets.
This attraction allows MagnaCat to separate and selectively purge the oldest, most contaminated particles because they have accumulated the most metals and are the most magnetic. This reduces the average age of the e-cat inventory and improves its activity and selectivity without increasing the fresh catalyst makeup rate.
The degree of attraction to a magnetic field is referred to as "magnetic susceptibility."
MagnaCat process
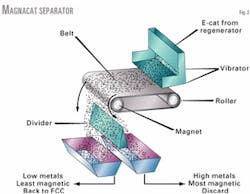
Fig. 2 shows the MagnaCat process. A roller magnet assembly separates e-cat into "most-magnetic" and "least-magnetic" cuts. E-cat is continuously fed onto a moving belt that has a high-field-strength permanent magnet roller at the far end of the assembly.
As the e-cat passes through a magnetic field generated by the roller, catalyst with higher magnetic susceptibility is retained on the belt. Catalyst with lower magnetic susceptibility is carried off the end of the belt where it falls into a chute.
As the belt turns around the roller and moves away from the magnetic field, magnetic catalyst retained on the belt drops into a separate chute. A third catalyst chute can be provided for large particles (primarily refractory and agglomerated catalyst) that travel a significant distance from the end of the belt.
The magnetic catalyst is referred to as "magnetic reject," and consists of catalyst that is substantially older and higher in metals than the equilibrium mixture.
Catalyst not retained on the magnet is called "recycle," which recycles back to the FCC regenerator. This material is younger and lower in metals than the equilibrium mixture.
Any oversized particles that are collected in a third chute are added to the reject stream for disposal.
If catalyst addition rate is held constant, the average catalyst metals level will drop and activity and yield selectivity will improve. If catalyst addition is reduced, catalyst metals levels and activity will remain essentially constant.
In most cases, MagnaCat usually operates between these two modes to provide optimum improvements in catalyst management and product yields.
KPI project
KPI commissioned an RFCC unit at its Chiba, Japan, refinery in 1994. The unit was originally rated for a throughput of 20,000 b/sd. Gradual improvements in operations and minor debottlenecking expanded throughput to about 30,000 b/sd.
The normal feedstock is a mixture of moderate-pressure hydrocracker bottoms and untreated vacuum residue from Middle East crudes. The RFCC unit has the flexibility to maximize either gasoline or middle distillate product yields.
In 1998, KPI contracted Kellogg Brown & Root (KBR) to design a MagnaCat unit based on 20 ton/day catalyst processing capacity.
Flow description
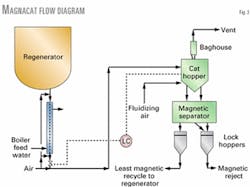
Fig. 3 shows a simplified flow diagram of the KPI facility. A slipstream of e-cat is withdrawn from the regenerator bottom and cooled against boiler feed water in a catalyst cooler.
From the cooler, catalyst is conveyed via plant air to the magnetic separator feed hopper. A valve regulates the catalyst flow so that a constant level is maintained in the feed hopper.
The magnetic separator segregates the e-cat into two fractions. Blow cases transport the separated catalyst. The most-magnetic catalyst is sent to the e-cat storage hopper and the least-magnetic fraction is returned to the regenerator. The most-magnetic fraction also contains large, oversized particles or pieces of refractory that are separately recovered by the magnetic separator and combined with the reject catalyst.
Magnetic separator performance
The KPI MagnaCat unit started up in September 1999. During the first several months of operation, KPI and KBR closely monitored the magnetic-separation performance. During this time, KPI kept the fresh catalyst addition rate constant relative to pre-MagnaCat operation. Unit feed rate, however, was increased by 22% slightly before implementation of MagnaCat, so that on a pound/barrel basis, catalyst additions were reduced by about 24%.
To quantify separation performance of the MagnaCat unit, sample sets of RFCC e-cat, magnetic reject, and recycle catalyst were collected. All samples were analyzed for metals content, catalyst activity, and selectivity using micro activity testing (MAT).
For the first 5 weeks of operation, average metals content in the reject catalyst was higher than in the recycle catalyst by 640 ppm nickel, 733 ppm vanadium, and 2,285 ppm iron.
At MagnaCat startup, differences in the reject and recycle fractions were pronounced. As the RFCC catalyst inventory was processed multiple times through the unit, the differences became smaller. This indicated the removal of old, metals-laden, deactivated catalyst particles.
In 33 days of operation, approximately three times the inventory had been processed. By that time, the RFCC experienced the full effect of catalyst treating. From a difference of nine MAT numbers at startup, there was a gradual decline to about three numbers.
Throughout this period, however, inventory catalyst properties were steadily improving.
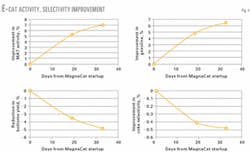
Fig. 4 shows MAT activity and selectivity improvements for the e-cat. The catalyst exhibited better gasoline yield, bottoms upgrading, and coke selectivity. These improvements occurred despite a 24% reduction in fresh catalyst addition.
Improved activity maintenance
KPI compared catalyst activity maintenance with the pre-MagnaCat operation after 1 year of operation.
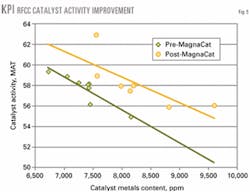
Fig. 5 shows MAT activity vs. nickel and vanadium content with and without MagnaCat. Table 1 provides a comparison of e-cat activity and metals level for pre- and post-MagnaCat operations.
With MagnaCat, catalyst activity is 2.2 numbers higher at a constant metals level. Alternatively, KPI can maintain constant activity with an additional 717-ppm total nickel plus vanadium on the catalyst.
RFCC improvements
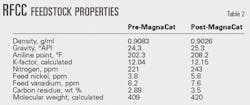
KPI performed detailed mass-balance calculations during control periods several months before and several months after installing the processing unit. Table 2 shows RFCC feed properties. Although the post-MagnaCat feed has a higher API gravity, the levels of nickel and carbon residue are all higher.
Table 3 shows RFCC operational parameters pre- and post-MagnaCat control periods. After installing the MagnaCat in September 1999, KPI moved forward with an existing plan to increase RFCC feed rate. By December 1999, RFCC charge rate was increased by 4,510 b/d and the amount of vacuum residue in the feed blend was also increased by about 10%, resulting in a 0.6 wt % increase in carbon residue content (Table 2).
One primary benefit is reduced production of coke and gas in the RFCC unit. Yields for the post-MagnaCat control period (Table 3) show that significantly less coke per barrel of feed was produced, despite higher feedstock carbon residue content.
KPI used a simulation model to predict yield performance of its RFCC based on feed quality, catalyst metals, and operating conditions. The model accurately predicted actual dry gas and coke yields before MagnaCat startup. Following startup, however, there was a distinct overprediction.
This indicates that MagnaCat lowered gas and coke yields by modifying e-cat properties in a way the model did not recognize. The unrecognized factor may be nickel on the oldest catalyst, which tends to be "clumped" rather than dispersed throughout the matrix. "Clumped" nickel is active towards the dehydrogenation reactions that produce hydrogen gas and coke.
Based on unit yield observations, MagnaCat reduced coke 5%-11% and dry gas 10%-16%. Maximum RFCC unit throughput increased by about 10% due to these coke and dry gas reductions. Improvements in catalyst circulation that occurred as MagnaCat removed oversized particles from the catalyst inventory helped increase throughput capacity.
RFCC yield shifts
KPI observed a shift in liquid product yields as the RFCC catalyst inventory reached a new equilibrium condition.
Consistently to quantify this shift, KPI compared unit yields from a post-MagnaCat control period when the unit was limited by the wet-gas compressor to a simulated yield pattern using the computer model tuned to pre-MagnaCat performance. The simulation was based on constant feed rate, feedstock properties, and fresh catalyst makeup rate. KPI adjusted severity conditions to drive the unit to the wet gas compressor limit.
Table 4 shows the results of this study.
Without MagnaCat, catalyst activity was lower by 2.1 fluid activity index (FAI) numbers (similar to MAT activity) and catalyst nickel and vanadium were higher (Fig. 5). The riser top temperature had to be lowered by 2° C. to stay within the wet-gas compressor limit.
Gasoline and C4 yields were lower and heavy bottoms yields were higher, resulting from lower temperature and catalyst activity. Regenerator air use was also higher and the catalyst cooler duty was higher due to higher coke yields without MagnaCat.
Results in Table 4 show reduced air consumption and catalyst cooler duty for a constant amount of vacuum residue in the feed.
KPI performed another exercise to show the benefits of lower coke selectivity when the cat cooler duty and air blower are limiting. Table 5 shows the results of an analysis to determine the increased amount of vacuum residue that can be processed in the RFCC feed blend with the unit operating against its air blower limit.
The model projected that an additional 780 b/d of vacuum residue at constant feed rate could be processed. This benefit results from reduced coke make with the RFCC unit constrained by air blower and catalyst cooler limitations.
The difference in e-cat nickel and vanadium between the actual yields and model prediction in Table 5 is less pronounced than the study summarized in Table 4. This is because the RFCC feed for the actual post-MagnaCat case in Table 5 contains 13% more metals due to the increased resid content. F
References
- Johnson, T.E., Goolsby, T.L., Miller, R., Fujii, F., Hara, M., Kowalczyk, D.C., and Campagna, R.J., "Successful Implementation of MagnaCat Technology at KPI's Chiba Refinery," Japan Petroleum Institute, Tokyo, Oct. 19-20, 2000.
- Johnson, T.E., Goolsby, T.L., Silverman, M.A., Moore, H.F., and Kowalczyk, D.C., "Catalyst separation technology improves FCC gasoline yields," OGJ, June 15, 1998, p. 65.
- Johnson, T.E., Goolsby, T.L., Silverman, M.A., Moore, H.F., and Kowalczyk, D.C., "Developments in MagnaCattrademark Technology," NPRA Annual Meeting, San Francisco, Mar. 15-17, 1998.
- Goolsby, T.L., Moore, H.F., Kowalczyk, D.C., Johnson, T.E., Zampieri, M.L., and Bussey, B.K., "A new approach to FCC unit optimization," Petroleum Technology Quarterly, Autumn, 1997, p. 29.
- Goolsby, T.L., Moore, H.F., Kowalczyk, D.C., Zampieri, M.L., and Bussey, K.B., "FCC unit optimization using the MagnaCattrademark process," NPRA Annual Meeting, San Antonio, Mar. 16-18, 1997.
- Kowalczyk, D.C., Campagna, R.J., Hettinger, W.P., and Takase, S, "Magnetic separation enhances FCC unit profitability," NPRA Annual Meeting, San Antonio, Mar. 17-19, 1991.
The authors
Rik B. Miller is a product and development manager for fluid catalytic cracking at Halliburton KBR. As development manager, his primary responsibilities are to direct and perform FCC technology development. As product manager, his responsibilities include marketing of KBR's FCC technology. Miller has more than 20 years' experience in a variety of refining processes. Before joining Kellogg in 1992, he worked for Unocal's Science & Technology Division, Brea, Calif. Miller received his BS (magna cum laude) in chemical engineering from the Georgia Institute of Technology, Atlanta.
Terry L. Goolsby is the manager of MagnaCat technology at Halliburton KBR. Goolsby has more than 22 years of experience in a variety of refining processes. Before joining Halliburton KBR in 1997, he worked for Ashland Petroleum Co.'s research and development department, Catlettsburg, Ky. Goolsby received his BS in chemistry and biology from Western Kentucky University, Bowling Green, Ky., and his MS in organic chemistry from Marshall University, Huntington, W.Va.