Processes combine to assist acid-gas handling, reinjection
From a paper presented at the GPA Gulf Cooperation Council technical conference, May 23, 2001, in Abu Dhabi.
TotalFinaElf and Institut Français du Pétrole (IFP) have been working for several years to develop less-costly technologies to handle high-H2S sour gases, especially those intended for bulk acid-gas disposal projects.
Acid-gas reinjection for disposal is important for greenhouse gas and sulfur emissions. Also, a diminishing sulfur market does not always justify Claus-type sulfur recovery.
The Elf Activated MDEA process, from TotalFinaElf, is a cost-effective way to meet a wide range of situations, from complete CO2 removal to bulk H2S and CO2 removal, even for acid-gas reinjection projects.
And an upstream acid-gas preextraction technique, called Sprex, originally patented by IFP and jointly developed with TotalFinaElf, offers a synergistic combination with the Elf Activated MDEA process for bulk H2S-rich acid-gas disposal projects.
The companies have jointly developed a third process, Hybrisol, a hybrid solvent acid-gas-removal process that integrates dehydration and NGL extraction to produce sweet, dry pipeline gas, sweet NGLs, and dry pressurized acid gases.
Process development
When Elf Aquitaine, before becoming TotalFinaElf, decided in the mid-1950s to produce gas from the Lacq field in southwest France, it faced several challenges because the field was the first highly sour, high pressure, and high-temperature gas field produced.
High performance was important to remove the large quantity of acid gases from raw gases containing more than 15% H2S and up to 10% CO2. Also, new steel alloys had to be developed to resist the highly corrosive fluids.
The technology first selected was the so-called SNPA-DEA process that exclusively used the reactive solvent diethanolamine (DEA).
The process was improved by nearly doubling DEA solution concentration. This reduced solvent circulation and operating costs while controlling corrosion without inhibitors.
The continually improved processes were implemented in several sweetening units between 1957 and 1968, eventually reaching a sales-gas production capacity of 25 million std. cu m/day.
However, with declining in production of the Lacq field and constantly increasing energy costs, new processes were needed that were better suited than DEA to a new competitive gas market. Fortunately, existing units and equipment-made available by the reduction of Lacq gas production-conveniently enabled conversion, remodeling, and testing new solvent formulations, absorber internals, and processing techniques on an industrial scale.
The new processes then developed were based on the use of tertiary methyldiethanolamine (MDEA) and a flash-procured rich amine-regeneration system. This significantly reduced the energy consumption of the amine reboilers.
Since 1977, MDEA has been used by Elf Aquitaine for deacidification of gases that do not require total CO2 removal.
The MDEA process allows control of CO2 slippage from the absorber by proper choice of absorber internals and operating conditions. This makes it suitable for many different applications and allows tailored amounts of CO2 in the product gas while making H2S-richer acid gas to Claus sulfur-recovery units.
Since 1986, an MDEA process has been used at Lacq to remove H2S from raw, high-pressure sour gas streams to produce H2S-rich streams used for thio-organic chemical synthesis.
The MDEA process for selective H2S removal has been implemented in more than 20 units worldwide, either operated by the TotalFinaElf group or other operating companies under license.
Also, the needs for total acid-gas removal and the ability to produce residue gas with less than 50 ppm CO2 subsequently led to the development of the Activated MDEA process with the removal performance of DEA and significantly reduced energy consumption.
Today, a series of chemical activators used with MDEA, in a split semiregenerative process scheme, offers the most cost-effective answer to complete or controlled acid-gas removal from sour to very sour natural gases.
Bulk removal
For a long time, TotalFinaElf has also been interested in the bulk removal of acid gases, notably in gas fields operated in the Far East with more than 40% CO2, as well as in the Caspian Sea region for natural gas and associated gases containing more than 20% H2S with CO2.
The world sulfur market today is saturated, but CO2 emissions as a greenhouse gas still needs to be drastically curtailed. This has led to serious consideration of acid-gas cycling or disposal by reinjection.
That means technologies other than the classical aqueous amine-based ones will need more-favorable attributes. These include the delivery of acid gases in a dry state and at higher pressures to reduce recompression power, facilitate reinjection system design, and avoid exotic materials.
TotalFinaElf and IFP have now embarked upon a comprehensive program to develop new gas-treatment technologies better to suit the bulk acid-gas removal destined for reinjection. Pretreatment techniques and hybrid solvent processes are seen as the most cost-effective solutions.
Activated MDEA
When the MDEA process was developed in the mid-1970s, it was principally destined for the sweetening of gases which did not require complete CO2 removal or required removal of only a controlled part of the CO2.1 Typical applications of the selective MDEA process are:
- H2S removal from gases already at or close to the allowable CO2 specification.
- H2S removal from gases in which CO2 is not important (i.e., fuel gas).
- Production of an H2S-rich (i.e., CO2 poor) acid gas for Claus-type sulfur recovery or thio-chemical synthesis.
MDEA is used for selective H2S removal because, unlike DEA, MDEA does not react directly with CO2; thus, CO2 absorption kinetics can be controlled by the slow reaction of CO2 with water.
For complete CO2 removal along with H2S, the more-reactive DEA has been traditionally used but with a serious energy-consumption handicap in its regeneration step. Ever-tighter cost constraints imposed on gas processing have led to development of a new MDEA process for complete acid-gas removal from sour gases with a substantially lower regeneration energy consumption than conventional DEA.
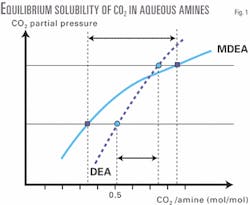
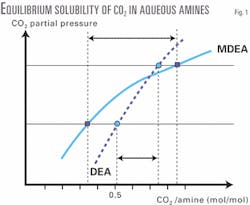
The two main factors affecting a solvent's performance for CO2 absorption and its ease of regeneration are solubility and reactivity. While reactivity of CO2 in MDEA is lower than in DEA, its solubility in MDEA is more strongly influenced by CO2 partial pressure than its solubility in DEA. This can be shown by the slopes of the equilibrium solubility curves (Fig. 1).
The basic concept for a new process is to take advantage of the slope of the equilibrium solubility curves of CO2 in aqueous MDEA solutions in order to liberate a maximum amount of the acid gas from the solution by simple physical pressure let-down flash. This reduces the thermal regeneration duty substantially.
For example, the MDEA solution releases almost twice as much CO2 as the DEA solution by a pressure letdown from 10 bar to 2 bar (Fig. 1). In addition, the equilibrium solubility of H2S in aqueous MDEA exhibits identical behavior to CO2, allowing equivalent amounts of H2S liberation by pressure letdown.
Unfortunately, MDEA reacts slowly with CO2. That is acceptable for selective H2S and controlled CO2 removal, but it is a handicap for complete acid-gas removal, especially when a substantial quantity of CO2 must be removed.
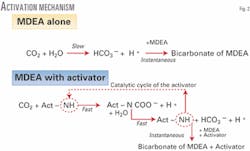
To overcome this kinetic obstacle, activators were sought among secondary amines with high reaction speeds with CO2 to blend into the otherwise desirable MDEA solvent. Fig. 2 shows the reaction mechanism.
With MDEA alone, the transformation of CO2 into bicarbonate is a slow process, while the reaction of the carbonic acid with MDEA is instantaneous. With an activator in the MDEA solution, the transformation to bicarbonate via a first-step formation of a carbamate is made faster.
Amines were selected with carbamate formation rates that could substantially activate the CO2-MDEA reaction and determine the concentrations required to optimize solvent efficiency. Several activators were selected, taking into account commercial availability, cost, and impact on the environment.2
Another process improvement over the conventional DEA process is the introduction of secondary, semilean solvent circulation (Fig. 3).
The rich amine solution after letdown through an hydraulic turbine is divested of coabsorbed light hydrocarbons in a first flash drum, as in the conventional process. It is then further expanded to low pressure in a second flash drum partially to liberate CO2 and H2S.
The greater part of the rich amine thus partially regenerated is returned to an intermediate level of the absorber as a semilean solvent. This second solvent loop is particularly economic because it reduces the thermal regenerator load and consumes only pumping energy.
Thoroughly regenerated, virtually H2S and CO2-free amine is required when H2S is present and the treated gas specification calls for the removal of H2S to less than 3.0 ppm, or for the removal of CO2 to less than the 1-2 vol % range. This is done in a conventional thermal regenerator returning a small flow of the leanest amine to the top section of the absorber.
In 1990, the new solvent and regeneration system were tested on an existing DEA unit at Lacq, which was then converted to the then-called Elf Activated MDEA process.3 4 This process was also used in several other locations in the North Sea such as at Sleipner West for CO2 removal and Elgin Franklin for controlled CO2 removal.
Different activators have been selected and patented to suit each specific case.
Process performance
The performance of the Elf Activated MDEA is closely related to site-specific treatment conditions, notably feed-gas composition and treated-gas specifications.
The following are practical rules-of-thumb to highlight the general interest of regeneration by flash:5
- The greater the H2S or CO2 partial pressure in the feed gas, the greater is the efficiency of the flash. In the case of the Lacq plant, the acid-gas partial pressure of the feed is about 15 bar compared to a total pressure of 2 bar in the flash drum. This gives a ratio of 7.5 (acid-gas partial pressure/flash pressure). In general, the advantage of flash starts above a ratio of 3.0.
- The overall energy consumption of the Activated MDEA unit increases as the treated gas specification becomes more stringent because the amount of lean totally regenerated amine from the thermal regeneration increases. In some CO2-only cases, it may even be possible completely to eliminate the thermal regenerator.
As a consequence, the Elf Activated MDEA process is well adapted to the treatment of high pressure and very sour gases where the advantages of flash-procured regeneration will be maximized.
Activated MDEA case study
The following is an example of the Activated MDEA process used for the treatment of high-H2S sour gas. It involves a plant operated by Elf in the 1970s in Alberta.
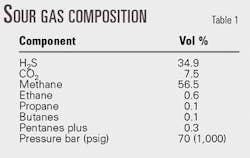
The original plant design used a conventional 30 wt % DEA solution that was considered state-of-the-art at the time the plant was built. Table 1 shows the gas composition on a dry basis.
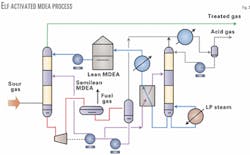
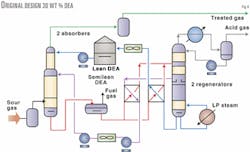
The treated gas had to meet typical pipeline gas specifications, i.e., less than 4 ppmv H2S and less than 2.0 vol % CO2. The acid gas liberated is sent to conventional two-stage Claus units followed by a Sulfreen tail gas-treating unit. Fig. 4 illustrates the original amine sweetening unit's process flow scheme. There are two identical trains, each equipped with two high-pressure absorbers and two regenerators.
The raw sour gas capacity of each train is 162 MMscfd or 4.6 MMscmd for a sulfur production of more than 2,000 tons/day. The reboiler duty for each amine train was 135 Mw (460 MMbtu/hr), roughly equivalent to the heat energy produced by the downstream Claus unit as low-pressure (LP) steam.
The original design was revised to reflect current state-of-the-art amine technology, using 48 wt % activated MDEA solution while retaining the same process scheme using a conventional double split flow design with thermal regeneration. This results in a decreased amine solution circulation rate and thus reduced reboiler duty. The reboiler duty is reduced to 91 from 135 Mw.
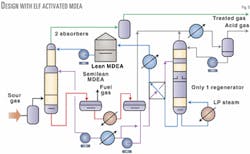
Fig. 5 shows the design of the amine units using the Elf Activated MDEA process with the addition of partial regeneration by flash.
In this case, only a third of the total amine solution circulation is sent to thermal regenerator; therefore, the reboiler duty is reduced to only 46 Mw. This uses only one third of the Claus unit's steam production, and only one regeneration column is needed for each train. That could result in additional capital cost savings.
The quality of the treated gas is identical to the original design, and the acid gas produced still has a very good quality with a hydrocarbon content of less than 1.0 vol %. The latter specification is important for charging a Claus unit but is equally important for acid-gas cycling or reinjection.
Table 2 summarizes the stepwise improvements achieved.
Acid-gas removal for cycling, disposal
Environmental concern due to greenhouse gas emissions has given ever-rising importance to the reinjection of CO2 removed from natural gases, either for enhanced oil recovery (EOR) or just simple disposal to a depleted reservoir to avoid atmospheric venting.
Moreover, with the growing acceptance of H2S reinjection as an alternative to costly sulfur recovery to a diminishing sulfur market, many very sour gas wells can now be reconsidered as exploitable. Many of these wells had been shut-in to reduce production costs.
In any case, acid-gas reinjection to a disposal reservoir could make exploitation of sour-gas reserves attractive even in a flat-gas-price scenario. Because H2S and CO2 are reinjected underground, there are no CO2 or sulfur emissions to the atmosphere.
Acid-gas removal from marketable hydrocarbons is the first step in sour gas production, and several acid-gas-removal processes are available.
For maximum versatility and economic benefit, the overall process scheme should have:
- High capacity for acid-gas removal with minimum hydrocarbon coabsorption.
- Easy regeneration by pressure letdown with minimal thermal input, as coproduced heat energy of the Claus unit is no longer available.
- Liberation of acid gases at some pressure and preferably cold and dry.
- Possibility of adapting regeneration pressures to interstage acid-gas compression.
Physical solvent processes give some, but not all, of the above qualities. The Selexol process has a number of industrial references, most of them for synthesis gas deacidification and some for natural gas treatment.6-8
A methanol-based refrigerated-solvent process such as Ifpex-2 from the Ifpexol technology matrix of IFP is also a good contender.9
Physical solvents, however, have a high affinity for hydrocarbons, and the separated acid-gas stream contains large quantities of valuable hydrocarbon products.
Chemical solvent processes generally have a higher energy requirement than physical solvent processes, but do not absorb hydrocarbons. Among these, the Activated MDEA process from TotalFinaElf, discussed earlier, which removes H2S completely and CO2 as required, has a low energy requirement thanks to the ability of the Activated MDEA to liberate the bulk of the acid gases in a simple flash (flash procured regeneration).
With the exception of acid-gas dryness and pressure, the Elf Activated MDEA process meets all of the above-listed characteristics for reinjection, most remarkably the liberation of acid gases without hydrocarbons.
This not only reduces sales-gas shrinkage but also reduces recompression flowing capacity.
The very low hydrocarbon content of the acid gas produced by the Activated MDEA process is another factor in the wellhead pressure requirements because hydrocarbons reduce the density and water solubility of the pressurized acid-gas fluids.
The only disadvantage of the Activated MDEA process is the liberation of the acid gases at low pressure.
Hybrid solvent processes mix a physical solvent with a chemical solvent, combining some of the advantages of physical solvent processes with those of chemical solvent processes.
The Sulfinol process is often used in sour-gas deacidification. Its energy requirement is relatively low, but hydrocarbon coabsorption is higher than that of an amine process.
The cost of acid-gas removal strongly depends on feed-gas acid content and the downstream acid-gas compression reinjection network. The reinjection well's completion design is strongly influenced by the acid-gas quality. In the case of very sour gas, the combination of a bulk-removal step ahead of the final sweetening unit can reduce the overall acid-gas removal cost.
If the acid gas is reinjected, the bulk-removal process should be able to preextract or recover acid gas in liquid phase and at high pressure for pumping to reinjection pressure.
Bulk H2S removal for disposal
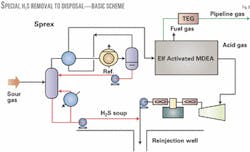
The proposed process for treating very sour gases (i.e., with an acid-gas content greater than 20%) with reinjection of the separated acid gases to a disposal reservoir incorporates a special patented H2S preextraction step upstream of the Activated MDEA acid-gas removal process.
In this upstream step, called Sprex,10 a substantial amount of the H2S and some of the CO2 are "preremoved" from the wet raw gas as a pumpable liquid stream. This liquid will essentially contain by solubility all the water of saturation that comes with the inlet raw gas.
It will also contain some of the incoming hydrocarbons. Fig. 6 shows this special preextraction and Activated MDEA combination process.
The dried gas leaving the Sprex contactor is cooled through a gas-gas heat exchange, gas-condensate heat exchange, and then through a refrigerated chiller before being passed to a low-temperature separator (LTS) drum operating essentially at line pressure.
Since the Sprex liquid removes most of the water from the feed gas by solubility, no further free-water condensation is expected in the chilling train. In practice, however, some hard-piped methanol injection would be incorporated in case upset trace free water might appear.
No methanol recovery is needed due to the negligible amount of-and only intermittent-injection of methanol, if any. Any injected methanol is thus considered lost.
The refrigerated chiller may be replaced by any other refrigeration process, such as a turboexpander, if the inlet fluid is available at sufficient pressure.
The separated liquid stream is pumped through heat exchange and warmed before being recycled to the Sprex contactor. The H2S-rich recycle contains dissolved hydrocarbons, which are then stripped from the liquid in contact with ambient temperature feed gas.
Due to the recycle, the H2S partial pressure is substantially increased in the contactor, allowing a good portion of the incoming H2S to condense and drop out in its bottom section.
The H2S-rich mixed liquid stream or "soup" is taken to the suction of the disposal pump at line pressure and near ambient temperature. This soup also contains preextracted CO2, some of the hydrocarbons from the inlet gas, soluble water, and any methanol injected. Pumping liquid is more energy efficient than compressing vapor to reinjection pressure.
The gas stream leaving the LTS with the remaining acid gas is warmed through gas-gas heat exchange and then taken to the absorber of the MDEA unit.
The MDEA unit uses the Elf Activated MDEA process design. The selection of an activator (or none) depends upon the amount of CO2 to be allowed to slip into the pipeline gas. In this case, CO2 removal can be selective with savings on solvent loading and regeneration duty.
When the remaining acid-gas loads are still significant, the partial flash regeneration may be used to reduce solvent circulation and reboil duty (Fig. 3).
The treated gas leaving the MDEA unit requires downstream dehydration because pipeline-gas specifications require a minimum water dew point. This is best achieved with a conventional TEG dehydration unit, as the gas is very lean without condensable hydrocarbons.
Sprex performance
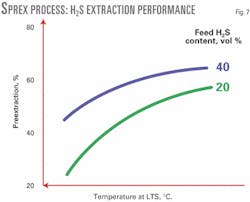
Acid-gas extraction performance of the Sprex process depends on the inlet fluid composition and the temperature in the LTS. The higher the acid-gas content of the inlet gas, or the lower the temperature in the LTS, the higher the preextraction performance (Fig. 7).
Hydrocarbon coextraction will increase with the acid-gas preextraction efficiency. For bulk removal of acid gas from very sour gases, in which very high acid-gas preextraction is achieved, more-sophisticated process schemes are being evaluated to maintain hydrocarbon coextraction at low levels.
The hydrocarbon shrinkage should be maintained below 3% in all cases, even when treating gases with a very high H2S content. For a temperature at the LTS of -30° C., an H2S preextraction of more than 70% may be achieved from a gas with 35% H2S. The CO2 preextraction performance of the Sprex process is somewhat lower than that of H2S.
Capital cost, energy comparison
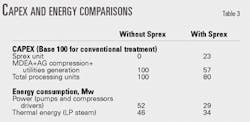
The Sprex soup is available at line pressure and in the liquid phase, thus considerably reducing the power consumption for reinjecting the acid gas. Since the MDEA process for the remaining acid-gas removal is now substantially reduced in its acid-gas-removal load, solvent circulation, equipment sizes, and investment costs are correspondingly reduced.
Table 3 summarizes the capital cost and energy savings with the Sprex process in the Canadian case described earlier, assuming that the separated acid gases are reinjected with a wellhead-injection pressure of 150 bar (2,140 psig).
The data in Table 3 refer to deacidification, acid-gas compression and pumping, and associated utilities generation; they are exclusive of field costs.
New gas-treatment technology
Nearly 6 years ago, IFP began research to find more versatile, if not better-performing, acid-gas-removal solvent mixtures than the straight aqueous amines or methanol used in its Ifpexol technology matrix.
IFP has acquired extensive know-how and experience in methanol use, first as a hydrate inhibitor in the commercially proven Ifpex-1 process for dehydration, and then as a physical acid-gas-removal solvent in the Ifpex-2 process. The company believes that methanol should play an important, if not dominant, role in a combined-solvent mixture to be used in an improved gas treatment technology.
This R&D effort resulted in the development of a new hybrid solvent process trademarked Hybrisol.
Total, before fully becoming TotalFinaElf, was interested in the new process and recognized it, as well as the Sprex technique, as a potential solution to some of the very sour gas-processing applications it was facing in the Caspian Sea region.
The Activated MDEA process is considered by TotalFinaElf as its acid-gas removal "workhorse" and still the most cost-effective solution to most acid-gas-removal applications. It has also become widely recognized, however, that for some site-specific and particular acid-gas disposal applications, limitations exist with MDEA alone.
Hybrisol has become the subject of further joint development because it combines the physical solvency attributes of methanol with the chemical reactivity and selectivity of secondary and tertiary amines.
Moreover, the presence of methanol as the physical solvent allows an enhanced removal of mercaptans and COS to the hybrid solvent.
This process11 also incorporates the most elegantly simple aspect of the well-proven Ifpex-1 technique for recovery of methanol from a decanted methanol-laden water stream emanating from a downstream NGL extraction and simultaneous chill dehydration system.
Placing the NGL extraction step downstream and not upstream of the acid-gas-removal process also assures H2S and CO2-free hydrocarbon liquids (NGLs).
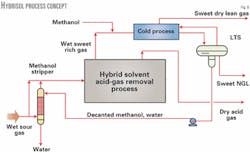
Fig. 8 shows a simplified concept of the Hybrisol process. It is applicable to most sour to very-sour, hydrocarbon-rich natural or associated gas feeds such as those found in the Caspian Sea area.
Water-saturated, sour hydrocarbon-rich feed gas is partially used as a stripping gas completely to strip the methanol from the decanted methanol-laden water stream from the LTS of the cold NGL extraction process.
This is the Ifpex-1 process.12 The small water stream removed from the bottom of the methanol stripper is virtually free of methanol but will contain some dissolved acid gases.
This represents the water of saturation that came with the wet feed gas. The vapor exiting the top of the stripper containing the recovered methanol is admixed with the by-passed gas and sent to the acid-gas removal process.
Since methanol is a significant part of the hybrid solvent, its presence in the feed gas to the absorber is only beneficial. The sweet gas leaving the acid-gas-removal process at basically ambient temperature is water saturated due to the last absorption zone being washed with aqueous amine/methanol solvent.
Very little hydrocarbon coabsorption is therefore expected. The exiting gas also contains methanol because its vapor pressure in the hybrid solution is rather high at the ambient temperature prevailing in the last absorption step.
The wet, sweet rich gas is next passed to a cold NGL-extraction process where external or autorefrigeration is used simultaneously to condense hydrocarbons and water in the presence of methanol as a hydrate and antifreeze agent.
The methanol makeup is injected just upstream of the first gas-gas heat exchanger of the cold NGL extraction process. The methanol-protected final cold mixture is then passed to the three-phase LTS for separation of a dry, sweet residue gas, sweet NGL, and a methanol-laden water stream.
The methanol content of this stream is adjusted as required to avoid hydrate formation or freezing within the cold process. The NGL stream will contain some dissolved methanol that is easy to recover by water-wash techniques described in other Ifpexol literature12 and not repeated here.
The water stream is pumped to the upstream methanol stripper previously described to complete the methanol recovery from the decanted water stream to the Hybrisol process.
Hybrisol process
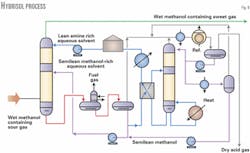
Fig. 9 is a simplified process flow diagram of the Hybrisol process for H2S and CO2 removal, which corresponds to the concept shown in Fig. 8. The wet sour gas also containing recovered methanol from the methanol stripper in Fig. 8 is taken to the bottom of the Hybrisol absorber column.
As the gas ascends the column, it is successively washed with methanol-rich, semilean aqueous solvent in the bottom section and then by amine-rich, lean aqueous solvent in the top section. All the H2S is removed to specification by the solvent.
CO2 is completely or selectively removed, depending on the nature of the chemical solvent chosen in the hybrid solvent mixture.
In order of highest-to-lowest volatility, the hybrid solvent consists of methanol, water, and a chemical solvent such as an amine or a polar solvent.
The water content of the mixed solvent is such that hydrocarbon coabsorption is minimized; yet solvency and reactivity with acid gases is optimized. The sweet gas leaving the absorber column is water saturated and contains equilibrium vapor pressure methanol.
The gas is passed to the cold process already mentioned for NGL and methanol and water removal from the residue gas product (Fig. 8).
Rich solvent leaving the bottom of the absorber column is regenerated by flash and thermal stripping steps, respectively. The first flash liberates most coabsorbed hydrocarbons that can be used as internal fuel gas.
A refrigerated, refluxing-type vent condenser can be used to limit the loss of methanol and reduce the H2S exiting in the gas. The second flash step to lower pressure starts to release CO2 and H2S from the hybrid solvent together with some methanol but very little, if any, water.
These flash vapors are combined with the stripper overhead and passed to an overhead cooling exchanger and refrigerated condenser for methanol recovery.
Part of the liquid phase separated in the second flash is a semilean solvent. It is recirculated to the lower section of the absorber after being mixed with most of the recovered flashed methanol distillate from the thermal stripper.
This mixture is a methanol-rich, semilean aqueous solvent that achieves readily the bulk acid-gas removal. The remaining part of the second flash semilean liquid is taken through heat exchange to the thermal stripper column.
The bottom of this column is reboiled at a temperature and pressure that results in only part of the methanol being distilled overhead with the acid gases. Essentially no water is distilled overhead.
Some controlled amount of methanol is left in the bottom lean aqueous amine solution to allow the thermal stripper to operate at some pressure appreciably greater than atmospheric without exceeding temperature limitations generally respected for amines.
The residual methanol content of the bottom lean solution also serves to reintroduce the appropriate concentration of methanol to the wet sweet gas upstream of the cold process.
Refrigeration is used for the final overhead condenser to minimize the methanol losses with the overhead acid gas.
The part of the condensed methanol containing some dissolved acid gases is used as reflux to the stripper column, and a controlled part is removed as a raw cold distillate. The cold distillate is reheated to ambient temperature by beneficial heat exchange (with the overhead for example) and passed to a flash drum operating at a pressure that allows liberated acid gases to be recycled to the overhead condenser.
The flashed semilean methanol liquid is then pumped to the semilean solvent circulation as desired. The acid gases leaving the stripper overhead system are dry and at a pressure slightly higher than would be possible with straight amine processes.
References
- Blanc, C., Elgue, J., and Lallemand, F., "MDEA process selects H2S," Hydrocarbon Processing, August 1981.
- Elgue, J., Peytavy, J.L., and Tournier-Lasserve J., "Recent industrial developments in natural gas sweetening by MDEA," paper presented at the 18th World Gas Conference, Berlin, 1991.
- Elgue, J., and Lallemand, F., "MDEA based solvents used at the Lacq processing plant," paper presented at the Gas Processors Association European Chapter meeting, London, Jan. 18, 1996.
- "Elf Activated MDEA: an important improvement in natural gas sweetening processes," 19th World Gas Conference, Milan 1994.
- Elgue, J., Peytavy, J.L., and Lallemand, F., "The Elf Activated MDEA process: new developments and industrial results," paper presented at the International Gas Research Conference, Cannes, 1995.
- Johnson, J.E., and Homme, A.C., "Selexol solvent process reduces lean, high-CO2 natural gas treating costs," Energy Progress, Vol. 4, No. 4 (December 1984).
- Shah, V.A., and Huurdeman, T.L., "Synthesis gas treating with physical solvent process using Selexol process technology," Ammonia Plant safety, AIChE, 1990.
- Epps, R., "Selective absorption of H2S and removal of CO2 using Selexol solvent," paper presented at the January 1996 GPA session, London.
- Minkkinen, A., Rojey, A., Charron, Y., and Lebas, E., "Technological developments in sour gas processing," Les Entretiens IFP, May 14, 1998, Rueil-Malmaison, France.
- Minkkinen, A., Benayoun, D., and Barthel, Y., US Patent 5,520,249, May 28, 1996.
- Rojey, A., Lebas, E., Larue, J., and Minkkinen, A., US Patent 5,782,958, July 21, 1998.
- Minkkinen, A., and Jonchere, J.P., "Methanol simplifies gas processing," paper presented at the 76th annual convention of the Gas Processors Association, San Antonio, Mar. 11, 1997.
The authors
François Lallemand is project leader for all gas-related works in the research and develolpment division of TotalFinaElf. He was previously process manager for Elf-EP and has spent most of his 27-year career in gas-related activities. Lallemand holds a chemical engineering degree from Ecole Centrale of Paris.
Ari Minkkinen is principal process advisor at Institut Français Pétrole (IFP) in France. He previously was with Total CFP in Paris as chief process engineer and before that with CE Lummus as a process design manager. He has been chairman of the Gas Processors Association-Europe and is at present a member of the editorial review board of the GPSA Engineering Data Book. Minkkinen holds post graduate degrees in chemical engineering from the Polytechnic Institute of New York.