Study determines best methods for calculating acid-gas density
A study on predicting acid-gas densities for the pressure and temperature ranges relevant to acid-gas injection schemes found that only one common equation of state gave unsatisfactory results.
Studied were the Soave-Redlich-Kwong (SRK), Peng-Robinson (PR), Patel-Teja (PT) equations as well as the effect of volume shifting on the SRK and PR equations. The study included pure hydrogen sulfide (H2S) and carbon dioxide (CO2) and mixtures of the two.
The study found that SRK equation was unsatisfactory for predicting densities over the entire range of conditions because errors for this equation were often greater than 10%.
The volume-shifted SRK is marginally satisfactory. The volume-shift dramatically improves the density in the liquid region but not in the supercritical region. The other four equations (PR, two volume shifted PR, and PT) exhibited errors less than 10%, except in the near critical region.
Knowledge of densities of these gases is important for designing acid-gas injection schemes.
Acid-gas injection
Acid gas is a byproduct of natural gas sweetening processes. It is toxic and environmentally problematic and must therefore be dealt with appropriately.
For large quantities of acid gas, operators typically use a Claus process to convert the H2S in the gas to elemental sulfur and release the CO2 to the atmosphere. But for small acid-gas quantities, injection has become an economical way to deal with the acid gas. Furthermore, acid-gas injection is a low-emission process, much lower than a Claus plant.
Western Canada currently has about 25 such projects in operation and several more in the planning stages. This disposal method also is starting to be looked upon favorably in other parts of the world.
One should note that acid-gas injection is not limited to small projects. Sulfur prices currently are low and elemental sulfur stockpiling may not be an option for sulfur producers. Limited space may force them to find alternatives for disposing of large quantities of acid gas by such means as injection.
Acid-gas injection is a simple concept. The acid gas from the amine regenerator tower is at a low pressure, typically less than 200 kPa (29 psi), and at about 50° C., the temperature of the overhead condenser. It also is saturated with water.
Multistage compressors compress the gas to the pressure required for injecting it into a deep reservoir. Surface pressure usually is substantially less than the reservoir pressure because of the hydrostatic head of the fluid being injected. Injection pressures depend on the injection zone and may be as great as 15 MPa (2,176 psi). Reservoir pressure can be 30 MPa or higher.
One key parameter in the design of an acid-gas injection scheme is fluid density.12 Needed are accurate density predictions for the vapor, liquid, and supercritical (dense-phase) regions.
This article reviews the available experimental data for acid-gas densities and examines several popular methods for density calculations. Density prediction methods that are phase specific will not be examined. For example, there are several correlations designed for estimating the liquid densities.3
In acid-gas injection, it is important to predict the density for all fluid phases. It is also important to have a well-behaved function as the fluid transverses the various phase regions.1 The liquid density correlations, therefore, are less useful for this application, even though they are accurate.
There is a large amount of experimental data available for CO2 and H2S densities. Therefore, this article includes only literature reviews. These compilations cover the pure component properties over the range of pressure and temperature of interest in this study.
For acid-gas injection, the temperature of interest if from 0° to 150° C. and the pressure of interest is from atmospheric to 30 MPa.
Pure CO2
Literature provides significantly more data on CO2 than for H2S, particularly for transport properties. One reason is that CO2 is considerably easier to deal with than H2S. In addition, CO2 has a much lower critical point, placing this interesting region in a range more accessible to experimenters.
The vicinity near the critical point attracts researchers because the physical properties in that region have properties which change dramatically with small changes in either temperature or pressure.
Span and Wagner4 provide the latest review of the thermodynamic properties of CO2. Their investigation is a through and critical review of the available experimental data. The tables generated use a highly accurate but complex equation of state. The study discussed in this article uses their tabulation to compare model density predictions.
Angus5 also thoroughly reviewed CO2 thermodynamic properties and formulated several tables, and Vukalovich and Altunin6 reviewed both CO2 thermodynamic and transport properties. Although their data sets are useful, they have been superceded by the newer tables of Span and Wagner.
Pure H2S
Goodwin7 extensively reviewed H2S thermodynamic properties. He used an advanced equation of state to construct a table of properties over a wide pressure and temperature range, which was used in the study discussed in this article.
The correlation for H2S has much less data than for CO2, but nevertheless, the Goodwin tables are probably the best currently available for H2S thermodynamic properties.
Binary mixtures
Robinson8 (see also Macrygeor- gos9) studied the phase behavior and volumetric properties of sour gas mixtures and reported the densities for three CO2 and H2S mixtures: 17.75%, 20.35% and 60.25% of H2S at 71.1° C. and pressures from 1.0 to 12.4 MPa. All these data are in the gaseous region with compressibility factors ranging from 0.95 to 0.45.
Kellerman10 in a more thorough investigation of the H2S plus CO2 binary system measured the densities of four mixtures: 6.07%, 9.55%, 29.33%, and 49.99% H2S. Temperatures ranged from -23.2° to 176.9° C. and pressures were up to 20.0 MPa. These measurements included both liquid and vapor regions.
The study discussed in this article used data from both Robinson and Kellerman.
Modeling acid-gas density
In process modeling, equations of state, mostly cubic equations of state, are the models of choice. This is particularly true for simulation processes for natural gas treatment.
Two popular cubic equations are the Soave11 modification of the Redlich-Kwong equation (SRK) and the Peng and Robinson12 (PR) equation of state. Literature contains additional modifications of these equations. In fact, commercial software packages often implement the modifications under the original names; therefore, the software user should be cautious.
Literature classifies PR and SRK equations as two-parameter, cubic equations. These equations can show significant deviations in predicted liquid density when compared to experimental data. Errors are typically on the order of 5-10%, although larger errors can be expected in the region near a critical point.
Recent attempts at improving liquid density calculations employ higher-order equations. For example, the Patel and Teja13 (PT) model has three parameters, and the Trebble14 15 (TBS) model has four parameters.
These higher-order equations rarely improve the vapor-liquid equilibrium compared to the predictions from the simpler two-parameter models, and therefore have not gained wide acceptance because of their added complexity.
This study focuses on PR, SRK, and PT equations of state for predicting acid-gas density in the vapor, liquid, and dense-phase regions. The accompanying box describes the component properties and summarizes the three equations of state.
Density
Equations of state relate the temperature, pressure, and specific (or molar) volume of a fluid. Cubic equations of state model the pressure of a fluid as a sum of an attractive and repulsive term. The equations are therefore in the form P = f(T,v).
Rearrangement of the equations to a volume explicit form produces a cubic, third-order, polynomial. The solution to a cubic equation result in one real or three real roots, of which two or all three may be equal.
For unsaturated fluids in the subcritical region, only one of the roots, the most thermodynamically stable, is physically meaningful. For sub-critical saturated fluids, two roots of equal thermodynamic stability are physically meaningful.
It is worth noting that high temperature, single-phase fluids may also produce three real roots, one or more being negative. Neglected are negative roots, or specific volume roots less than the co-volume of the equation of state, b (see box).
After one calculates the molar volume, v, Equation 1 (see equation box) provides the molar density, r~.
Furthermore, Equation 2 calculates the mass density, r, which is the normal definition of the density. In Equation 2, M is the molar mass (molecular weight) of the fluid.
Volume shifting
Volume shifting is a popular method for improving density predictions from cubic equations of state. In its simplest form, originally proposed by Peneloux,16 volume shifting is a correction to the calculated molar volume (Equation 3). In Equation 3, vEoS is the volume estimated from the equation of state and c is the volume-shift parameter, which in this case is a constant.
If one properly selects the volume shift parameter, then the corrected volume should be an improved estimate of the true molar volume. Peneloux suggested fitting the saturated liquid density at Tr = 0.7 to obtain the volume-shift parameter. Alternatively, one could use the volume-shift parameter as an adjustable parameter, which is fit by minimizing the error in the density prediction.
To apply this method to mixtures, one assumes that the c for the mixture, cmix, is the mole-fraction weighted average of the parameters for the pure components (Equation 4). In this equation, xi, is the mole fraction of component i, and ci is the volume shift parameter for component i.
Mathias17 noted that the volume-shift method of Peneloux improved the liquid density prediction only up to a reduced temperatures of about 0.85. To improve the prediction over the entire range Mathias proposed an extended correction procedure that begins with Equation 5. In Equation 5 the s is a volume-shift parameter and it is a constant, and d, the bulk modulus, is a dimensionless parameter which is defined by Equation 6.
In Equation 6, R is the universal gas constant, T is absolute temperature, and P is total pressure. This expression can be evaluated from the equation of state.
Finally the function, ƒc, was chosen such that the volume shifting procedure calculated the true critical point. For the PR equation, Equation 7 calculates this function, in which b is the co-volume from the equation of state.
For mixtures Mathais used the usual, simple mixing rule provided by Equation 8.
Others have proposed making the volume-shift parameter with different functions of temperature. This adds to the model complexity. In addition, a poorly constructed temperature-dependence can lead to thermodynamic consistency problems; for example, see Monnery.18
This study only examines the corrections proposed by Peneloux and Mathias. Values of c and s for CO2 and H2S used in this study are listed in the box.
Error estimation
The study examined six density calculation methods, as follows:
- The original SRK.
- The SRK with a Peneloux-type volume shift.
- The original Peng-Robinson equation.
- The PR equation with a Peneloux-type volume shift.
- The PR equation with a Mathias-type volume shift'
- The PT equation.
The box presents a complete list of parameters used in this study.
Equation 9 defines the error for a given point, as a percentage. In this equation, tabular data provide the "value" and an equation, calculated at the same conditions, derives the "estimate."
Equation 10 defines the absolute error and Equation 11 defines the average error, AE, expressed as a percentage. The NP is the number of points.
The average error can be a positive or negative value. The better fit, however, occurs when the average error is close to zero.
Equation 12 defines the absolute average error. The difference between the AE and the AAE is that positive and negative errors in AE tend to cancel each other, making the prediction look better than it may actually be.
The average absolute error can only have a positive value because of the absolute value function. It is a better indication of the "goodness of fit" than is the average error. A small AE and a relatively large AAE usually indicates a systematic deviation between the function (values) and the predictions (estimates).
Finally, Equation 13 determines the maximum error, MaxE. The maximum error gives the largest deviation of the model from the data values.
There are other methods for estimating the error of a model, but the ones discussed are sufficient for the purposes or this study.
Pure components
Table 1 lists the six regions for which the study evaluated the errors for the various density prediction methods.
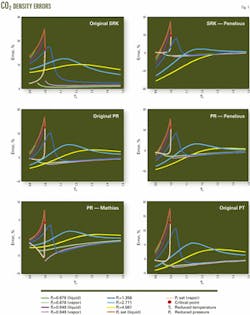
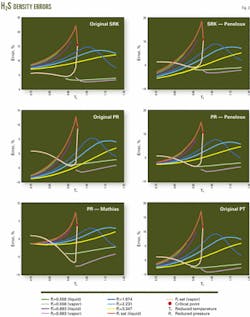
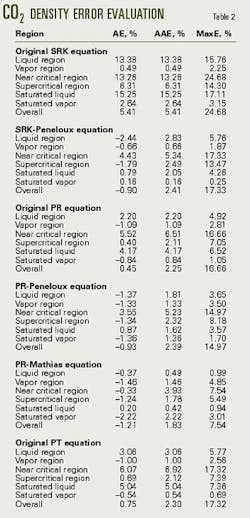
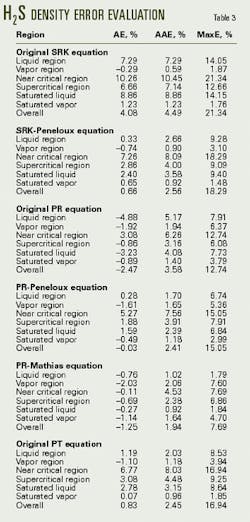
Tables 2 and 3 list the errors predicted for the density of pure CO2 and H2S, while Figs. 1 and 2 display a portion of the error data for each equation over the various regions.
These tables and figures show that the density predictions from the SRK equation are unsatisfactory. Although the overall average error is only about 5%, the maximum errors often exceed 15% and not only in the near critical region.
The PR equation is better at predicting the densities of these components than is the SRK equation, which is as expected. For H2S, the PT equation is a further improvement over the PR equation, again as expected.
For CO2, however, the PT equation does not improve the density predictions over the PR equation. As a check that the implementation of the PT was correct, the study set c to 0.3074, which reduces the PT equation to the PR equation. The calculated results obtained for this form of the PT equation were identical to those from the PR equation.
In all cases, the volume-shifting results in an improvement in the predicted liquid density. Overall, however, volume shifting does not always result in an improvement. For example, for CO2, the density predictions with the Peneloux-type volume shift of the PR equation actually are worse than the original PR equation. The reason for this is that the volume shifting results in worse predictions of the vapor density.
Mixtures
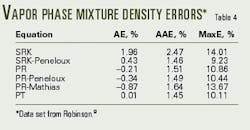
Table 4 shows the errors for predicting the data from Robinson.8 Because these data are only for the vapor phase they were not divided into regions for analysis. In general the errors for these mixtures are relatively small although a few points have larger errors.
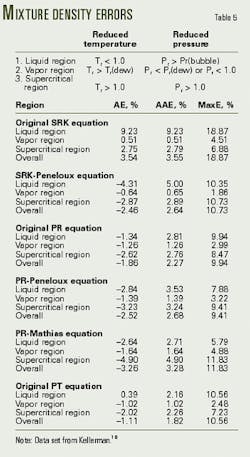
The data set of Kellerman10 is sufficiently large that it was examined in three regions, and Table 5 gives the definitions of these three regions and summarizes the errors for the six equations.
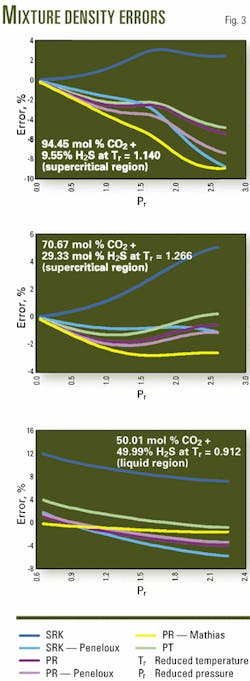
Figs. 3 presents plots of the errors for the predictions from the six equations for the various mixtures.
In general the observations for the pure components hold true for the binary mixture data. For example, the SRK equation predict unsatisfactory the density values.
The PR equation predicts the mixture data with acceptable accuracy, with the maximum error being less than 10%.
Although the volume-shift methods improve the predictions for the liquid density, the overall density predictions are only marginally improved.
Finally, the PT equation is an improvement over the PR equation when one considers the overall errors. The maximum error for the PT equation, however, is about equal to that for the PR.
Study results
The study concludes that the original SRK equation is not sufficiently accurate for predicting the density of acid gas. Although the average errors are acceptable, the maximum errors in the various regions exceed 10%.
The Peneloux-type volume shifting improves this equation significantly. For the pure components, only in the near-critical region do errors exceed 10%. For the mixture data, the maximum errors are typically less than 11%.
The original PR equation is accurate for predicting the densities of pure components. Overall, the average error for both the pure components is less than 5% and for the mixtures, less than 10%. Only in the near critical region do errors exceed 10%.
Volume-shifting the PR equations tends to improve density predictions in the liquid region. The predictions from the Peneloux-type volume shift have an overall error of 2.5%, and the errors from the Mathias-type are less than 2%.
The PT equation, although somewhat more complex, does not significantly improve the density calculations.
The observations for pure components are basically the same as for the mixtures examined. The SRK is unsatisfactory for predicting the density of these mixtures. The other five equations of state and modifications are all sufficiently accurate for engineering calculations, except in the region near a critical point.
References
- Carroll, J.J., and Lui, D.W., "Density, phase behavior keys to acid-gas injection," OGJ, June 23, 1997, p. 63.
- Carroll, J.J., and Maddocks, J., "Design considerations for acid gas injection," 49th Laurance Reid Gas Conditioning Conference, Norman, Okla., Feb. 21-24, 1999.
- Reid, R.C., Prausnitz, J.M., and Poling, B.E., The Properties of Gases & Liquids, 4th edition, New York: McGraw-Hill, 1987.
- Span, R., and Wagner, W., "A new equation of state for CO2 covering the fluid region from the triple-point temperature to 1100 K at pressure up to 800 MPa," J. Phys. Chem. Ref. Data, Vol. 25, 1996, pp. 1509-96.
- Angus, S., Armstrong, B., and de Reuck, K.M., International Thermodynamic Tables of the Fluid State - CO2, Oxford, UK: Pergamon Press, 1976.
- Vukalovich, M.P., and Altunin, V.V., Thermophysical Properties of CO2, translated from Russian, London: Collet's Publishers Ltd., 1968.
- Goodwin, R.D., H2S Provisional Thermophysical Properties from 188 to 700 K at Pressure to 75 MPa, Report No. NBSIR 83-1694, National Bureau of Standards, Boulder, Colo., 1983.
- Robinson, D.B., Macrygeorgos, C.A., and Govier, G.W., "The volumetric behavior of natural gases containing H2S and CO2," Petroleum Transactions, AIME, Vol. 219, 1960, pp. 54-60.
- Macrygeorgos, C.A., Phase and Volumetric Behavior in the Methane-CO2-Hydrogen Sulfide System, MS thesis, Dept. Chemical Engineering, University of Alberta, Edmonton, 1958.
- Kellerman, S.J., Stouffer, C.E., Eubank, P.T., Holste, J.C., Hall, K.R., Gammon, B.E., and Marsh, K.N., Thermodynamic Properties of CO2 + H2S Mixtures, GPA Research Report RR-141, Tulsa, 1995.
- Soave, G., "Equilibrium constants from a modified Redlich-Kwong equation of state," Chem. Eng. Sci., Vol. 27, 1972, pp. 1197-1203.
- Peng, D-Y., and Robinson, D.B. "A new two-constant equation of state," Ind. Eng. Chem. Fund., Vol. 15, 1976, pp. 59-64.
- Patel, N.C., and Teja, A.S., "A new cubic equation of state for fluids and fluid mixtures," Chem. Eng. Sci., Vol. 37, 1983, pp. 463-73.
- Trebble, M.A., and Bishnoi P.R., "Development of a new four-parameter equation of state," Fluid Phase Equil., Vol. 35, 1986, pp. 1-18.
- Salim, P.H., and Trebble, M.A., "A modified Trebble-Bishnoi equation of state: thermodynamic consistency revisited," Fluid Phase Equil., Vol. 65, 1991, pp. 59-71.
- Peneloux, A., Rausy, E., and Freze, R., "A consistent correction for Redlich-Kwong-Soave volumes," Fluid Phase Equil., Vol. 8, 1982, pp. 7-23.
- Mathias, P.M., Naheiri, T., and Oh, E.M., "A density correction for the Peng-Robinson equation of state," Fluid Phase Equil., Vol. 47, 1989, pp. 77-87.
- Monnery, W.D., Svrcek, W.Y., and Satyro, M.A., "Gaussian-like volume shifts for the Peng-Robinson equation of state," Ind. Eng. Chem. Res., Vol. 37, 1998, pp. 1663-72.
- Knapp, H., Döring, R., Oellrich, L., Plöcker, U., and Prausnitz, J.M., Vapor-Liquid Equilibria for Mixtures of Low Boiling Substances, D ECHEMA Chemistry Data Series Vol. VI, Frankfurt, 1982.
The authors
Tim Boyle is a reservoir engineer with PanCanadian Energy Corp., Calgary. He has experience in polymer, miscible, and thermal enhanced recovery projects. Boyle holds a BS in chemical engineering and is a member of the Association of Professional Engineers, Geologists, and Geophysicists of Alberta.
John J. Carroll is manager of simulation services for Gas Liquids Engineering Ltd., Calgary. He previously worked for Honeywell Hi-Spec Solution and was a research associate and lecturer at the University of Alberta in Edmonton. Carroll holds bachelor and doctoral degrees in chemical engineering from the University of Alberta and is a registered professional engineer in the provinces of Alberta and New Brunswick.