New method describes FCC catalyst selection for diffusion limited units
Recent advances in FCC short contact time operations coupled with heavier feedstocks have resulted in more catalytic crackers operating in a mass transfer limited regime. The rate of mass transfer-not catalytic activity-determines the reaction rate in these units.
These operations do not typically respond to independent variable manipulation for improved conversion and selectivity. For example, in many of these operations, bottoms conversion shows little response to increased riser outlet temperature or increased catalyst activity but often shows a large response to improved catalyst accessibility.1
Units operating with diffusion limitations will not respond to a classic catalyst selection methodology. Current state-of-the-art catalyst selection criteria are based on classic FCC catalysis in which the reaction rate is dependent primarily upon the number of active cracking sites in the riser.
Classic FCC catalyst selection paradigms typically do not improve mass transfer limited operations. In addition, certain laboratory performance tests cannot distinguish performance advantages between high accessibility and standard accessibility catalyst technologies.
New techniques can determine if the unit being optimized is mass transfer limited. Furthermore, new FCC catalyst technologies can shift most FCC units operating in the diffusion-limited regime into the activity-limited regime.
Refiners can use these guidelines to identify and improve conversion and yield selectivities for diffusion-limited operations:
- Analyze equilibrium catalyst accessibilities via the Akzo accessibility index (AAI) test and compare with unit performance data to define the response to accessibility.
- Identify the critical accessibility limit to determine whether the unit is diffusion limited. Where does the target unit operate with respect to the critical accessibility limit?
- Determine the minimum fresh catalyst accessibility based on the target unit's accessibility retention.
- Prescreen catalyst samples for minimum required accessibility using the AAI test.
- Use the best available deactivation protocol to rank the catalysts. The equilibrated catalyst properties should be tested in a small-scale fluidized bed reactor or circulating pilot plant with a representative feed.
- Use standard modeling or scale-up factors with refinery economics for final catalyst selection.
Akzo accessibility index
Akzo Nobel has developed the first commercial laboratory test capable of measuring the dynamic diffusion of hydrocarbon molecules in an FCC particle. The AAI test is a rapid screening test mainly based on liquid-phase diffusion of large organic molecules into the catalyst. No reaction is involved and the test only determines the catalysts' initial mass transfer characteristics.
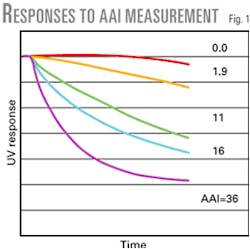
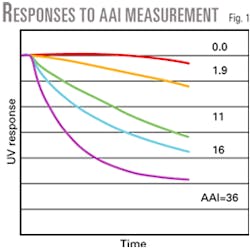
The test places the catalyst in a solution of dissolved petroleum, and monitors the solution's penetration using an on line UV spectrometer. The AAI is a relative measure of the penetration rate. Fig. 1 shows catalysts with a wide range of accessibilities.
Every FCC unit displays a critical accessibility limit-the point at which the unit enters a mass transfer limited operation.
Units operating below the critical accessibility limit experience a severe loss of conversion and bottoms destruction. This is because the high molecular weight, sterically hindered molecules cannot access the catalyst particle interior for cracking to occur. The loss of bottoms conversion is generally the first sign of a mass transfer-limited operation.
Root causes
Two factors determine mass transfer limited operations: unit design and feed quality. The combination of these factors defines the FCC's critical accessibility level. Catalyst technology and equilibrium catalyst metals concentration determine whether a given unit will operate above or below this critical accessibility value.
Unit design
Short contact time revamps provide substantial FCC yield improvements due to less dilute phase, nonselective cracking reactions. Conversion typically drops after a revamp and recovers through an increased catalyst-to-oil ratio and increased catalyst activity.
Some revamps, however, fail to respond as predicted due to mass transfer limitations. These revamps significantly reduce dilute phase contact time in the reactor vessel, which may result in a mass transfer limited operation.
Units that have never experienced mass transfer limitations can become diffusion limited after a short contact time revamp. This limitation can be overcome through proper catalyst selection.
Feed effects
Feed quality has a strong influence on mass transfer limitations. Catalyst particle accessibility is critical with higher concentrations of high molecular weight, sterically hindered molecules. The primary quality measurements impacting feed effects include feed density, distillation, and concarbon content.
Increasing feed density, residue concentration, concarbon content, and steric hindrance will eventually result in a mass transfer limited operation. Units operating with conventional contact times may also operate in a diffusion-limited regime while charging heavy feeds.
Equilibrium catalyst metals, accessibility
In an FCC unit, hydrothermal deactivation and metals deposition on the catalyst occur simultaneously with detrimental effects to the concentration, type, and accessibility of active sites. Hydrothermal deactivation and metals deposition can drastically modify pore shape and structure, which has a significant influence on the diffusion rate of molecules through the porous catalyst particle. The AAI test can detect changes in catalyst accessibility that influence hydrocarbon diffusion rate.
Examining equilibrium catalyst age fractions shows that, in most cases, catalyst accessibility as measured by AAI test decreases with increasing age; furthermore, nitrogen adsorption analyses show that a lower volume of micropores and mesopores and an increased average pore size accompanies the decreased accessibility.
Physico-chemical characterization, therefore, indicates that pores in the particle's outer and inner surfaces can change dramatically in an FCC unit. One must also consider the influence of hydrothermal deactivation and metals deposition when regarding the factors that cause changes in catalyst architecture.
Depending on feed characteristics, metals such as vanadium, nickel, sodium, calcium, and iron enter the FCC unit and deposit on the catalyst particles' outer surface. This can suppress active site accessibility by blocking the sites or pores. Nickel and iron tend to collect at the outer surface of equilibrium catalyst.
The different influences of iron and nickel on accessibility are due to the mechanisms with which these species "stabilize" on the FCC catalyst. In contrast to nickel, increased surface concentration of iron coincides with an appearance of iron-enriched nodules on the catalyst outer surface, as observed with scanning electron microscopy combined with energy dispersive x-ray analysis (SEM-EDX) or transmission electron microscopy (TEM).
In some cases, the particles form a glaze on the outer surface. Blockage of the catalyst particle's outer surface, as with iron poisoning, lowers catalyst accessibility and unit conversion. The complex mechanism of iron poisoning is not fully understood and is the subject of intensive ongoing investigation.
Factors-such as catalyst type, the type of iron compounds in the feed, unit conditions, and the presence of other poisons like sodium, calcium, magnesium, and vanadium-clearly influence iron poisoning. Iron poisoning occurs for all the common FCC manufacturing technologies.
Metals, such as vanadium and sodium, also deposit on the accessible catalyst sites; but, depending on the operational parameters, these species may migrate into the particle. The presence of steam and an oxidizing atmosphere enhances the mobility of these species.
Vanadium and sodium can destroy the zeolite pore architecture. Due to the high potential mobility of vanadium and sodium species and their "stabilization" on zeolite and alumina structures, however, the local concentration seldom gets high enough to form species that block the meso or macrosize matrix pores.
Recent advances in the understanding and application of the AAI test allows us to optimize catalyst accessibility. These advances lead to the successful commercialization of two new technology platforms that substantially increase fresh and equilibrium catalyst accessibility.2
Multiple units have shifted out of the diffusion-limited regime due to optimized catalyst accessibility. This provides refiners an additional degree of freedom for operational optimization.
Identifying critical accessibility
Measuring the accessibility of different equilibrium catalysts determines the presence of mass transfer limitations. These samples should span the normal range of equilibrium metals, surface area, and activity. Equilibrium metals include nickel, vanadium, sodium, iron, calcium, and potassium.
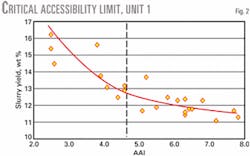
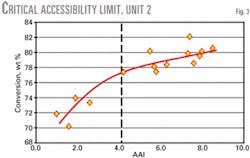
Lower conversion, slurry yield, and slurry density are typically the first indicators of a unit operating below its critical accessibility limit. Cross-plotting conversion and slurry yield vs. accessibility identifies mass transfer limitations.
An inflection point in the accessibility curve indicates if the unit has operated below its critical accessibility level. This inflection point defines the critical accessibility level. Operating below this critical accessibility limit results in a significant loss in unit conversion and profitability.
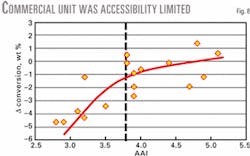
Figs. 2 and 3 present two commercial examples of mass transfer limited units. Both commercial operations were diffusion limited. The vertical dashed line indicates the critical accessibility limit.
These units experienced a substantial accessibility improvement after reformulating their base catalysts using the high accessibility technology, as shown by data to the right of the critical accessibility line. Both units experienced a significant improvement in conversion and bottoms selectivity.
Laboratory catalyst ranking
There are two approaches to ranking catalysts in the laboratory according to their accessibility: in the absence or presence of cracking reactions. The AAI test operates in the absence of catalytic reactions, and can easily rank catalyst parameters.
On the other hand, ranking catalysts in small scale or pilot plant cracking tests can address both catalyst activity and accessibility. The quality and applicability of the results depend on the type of unit and test protocol used.
AAI test
Knowing a catalyst's accessibility, accessibility retention of the catalyst in the target unit, and the unit's critical accessibility level can establish a particular unit's required fresh catalyst accessibility. Comparing fresh vs. equilibrium catalyst accessibilities can determine the accessibility retention of a particular catalyst technology. The most suitable catalyst for the target unit will fulfill the required fresh accessibility, accessibility retention, and preferred formulation.
Cracking tests
Cracking tests performed on a laboratory-deactivated catalyst reflect its activity and stability; furthermore, depending on the type of unit and test protocol, the results may also provide information about catalyst accessibility. To ensure proper catalyst ranking for diffusion-limited units, one must determine whether the catalyst selection protocol can distinguish accessibility effects.
Small-scale laboratory reactors. Small scale fluidized-bed reactors, such as fluid-bed simulation test (FST)3 and short contact time resid test (SCT-RT),4 are examples of laboratory scale tests capable of measuring the effects of catalyst accessibility.Pilot risers can also be sensitive to catalyst accessibility. This implies that resulting test data reflects the catalyst's kinetic and diffusion properties. If catalyst accessibility increases, the reduced diffusion limitations allow higher conversion and net bottoms conversion, for example.
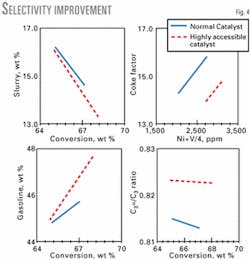
At constant conversion, increased catalyst accessibility results in lower coke, bottoms, and dry gas yields, while gasoline yield and LPG olefinicity increases. Fig. 4 shows these yield shifts in an FST unit. Improved selectivity corresponds to hydrocarbon diffusion in the catalyst particle.
Enhanced accessibility decreases the probability of secondary or slower reactions, such as gasoline overcracking, hydrogen transfer, and coke formation. Accessibility effects observed in FSTs and SCT-RTs have been established for laboratory deactivated and equilibrium catalysts with a wide range of accessibilities.
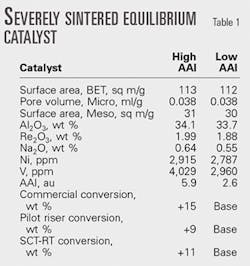
Table 1 shows the conversion response of a SCT-RT and a pilot riser vs. increased catalyst accessibility. When compared to diffusion-limited commercial FCC operation, the response in SCT-RT and pilot riser is similar in magnitude.
Contrary to the FST, SCT-RT, or pilot riser, a conventional micro simulation test (MST)5 or micro activity test (MAT)6 does not show a significant response to increased catalyst accessibility at a constant formulation.
When ranking catalysts in a laboratory for diffusion-limited FCC units, one must realize that the MST and MAT do not address catalyst accessibility. Laboratory deactivated and equilibrium catalysts demonstrate the inability of the MST and MAT to rank catalysts based on accessibilities.
To summarize, a realistic catalyst ranking for diffusion-limited units requires a laboratory testing protocol that is sensitive to both catalyst composition and accessibility. The conventional MST or MAT does not fulfill these requirements. One must consider the feed type and deactivation method in addition to the type of test unit.
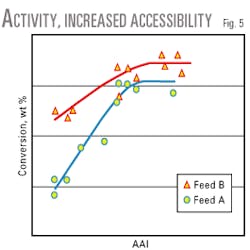
Feed effects. When ranking catalysts for diffusion-limited FCC units using laboratory tests, one should use a feed similar to that of the target unit. The test may be diffusion limited depending on the feed type and the degree of steric hindrance compared to a proposed catalyst.Fig. 5 shows the effect of feed type on the conversion of high accessibility catalysts. Feed A conversion increased with rising catalyst accessibility, whereas feed B conversion showed a weaker dependency on catalyst accessibility.
The AAI test demonstrates that feed A penetrates more slowly into the pores of the studied catalyst than feed B. An analysis using size exclusion chromatography (SEC) demonstrates that feed A contains a fraction of molecules with a larger average size than feed B.
The combined AAI and SEC analysis of these oils explains the observed sensitivity to catalyst accessibility. In this case, the standard laboratory analyses such as micro carbon residue, distillation, and metals content did not reveal the differences between feeds.
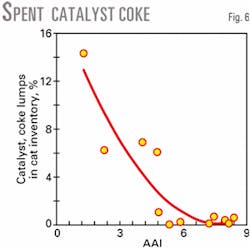
An interesting observation during laboratory testing of specific feedstocks is the appearance of catalyst and coke lumps in the spent catalyst. This may be a symptom of insufficient catalyst accessibility that prevents diffusion of bulky feed components into the catalyst pore system. As a result, carbonaceous deposits are formed at the external catalyst surface.
Fig. 6 shows an example of lump formation and its dependency on fresh catalyst accessibility. The percentage of lumps in the spent catalyst decreases with increasing fresh catalyst accessibility. This agrees with a decrease in reactor transfer line coking in a commercial unit after introducing high accessibility catalyst technology.
To summarize, the magnitude of unit response to catalyst accessibility depends on the feed. The recommended procedure for testing and ranking catalysts for diffusion-limited units is to use a feed that is representative of the target unit.
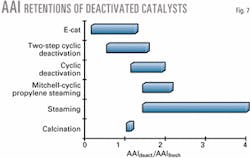
Lab simulation of deactivation. Since realistic catalyst ranking in the laboratory requires representative test units and feeds, one must also improve the methods currently used to deactivate fresh catalysts.With current sophisticated cyclic deactivation (CD) methods,7 8 many equilibrium catalyst properties-such as vanadium and nickel concentration profiles, metals activity, and zeolite and matrix surface areas-can be efficiently matched.
The accessibility, however, of laboratory deactivated catalysts shows that the architectures of equilibrium catalysts cannot be reproduced in the laboratory with current methods. This is especially true for steam deactivation, but also includes various CD and Mitchell impregnation methods.
These deactivation protocols yield higher deactivated accessibilities than for fresh catalysts (Fig. 7).
Equilibrium catalysts frequently show accessibilities less than those of the fresh catalysts.
We have developed a modified CD method to obtain a more realistic catalyst ranking and simulation of equilibrium catalysts in the laboratory. This new method can tailor deactivated catalyst accessibility over a wide range.
Representative laboratory deactivated catalysts will be produced in terms of accessibility, activity, metals concentration, surface area, and zeolite-to-matrix ratio.
This new method provides a substantial improvement in the accuracy of laboratory testing and catalyst ranking for diffusion-limited operations.
Commercial experience
A European refiner was consistently operating in a mass transfer limited regime (Fig. 8). The critical accessibility level for this unit was approximately 3.8. The refiner decided to conduct laboratory screening of high accessibility candidates.
The AAI test prescreened catalysts for acceptable accessibilities. Performance testing was then performed on the most promising candidates.
MAT testing failed to show any advantage for the high accessibility catalysts consistent with the observations for ranking these catalysts.
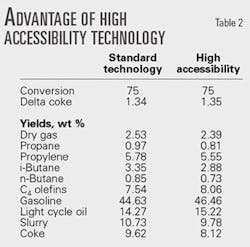
Table 2 shows the results of SCT-RT testing. Both tests indicated that the delta performance between the catalyst used in the unit and its high accessibility analog was large enough to warrant a commercial trial.
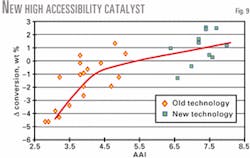
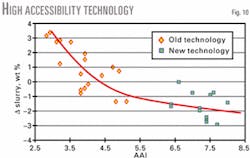
The commercial unit's accessibility after the catalyst change out increased to 7.5 from 3.0. Conversion increased by approximately 2.0 wt % while slurry yield was reduced by 1.5 wt % at constant feed quality and operating conditions (Figs. 9 and 10). An additional benefit was a substantial capacity for increased metals loading without negative impact to the yield structure.
References
- Foskett S.J., and Rautiainen, E., "Iron contamination in resid FCC," Hydrocarbon Processing, November 2001.
- Yanik S.J., Humphries, A., Pinto, R, and Fletcher, R.P., "Inventing the future in RFCC: Jade And Topaz Technology," Akzo Nobel Symposium 2001, Noordwijk, Netherlands, June 10-13, 2001, paper F-10.
- Pearce, J., Keyworth, D., Humphries, A., and Quinones, A., "Ultrashort contact time cracking and its simulation in the laboratory," presented at the AIChE 1998 Spring National Meeting, New Orleans, Mar. 8-12, 1998.
- Olthof, F.P., Smeink, R., Coopmans, J., and O'Connor, P., "Accessible catalysts for short contact time cracking," Fluid Cracking Catalysts, eds. Occelli and O'Connor, Marcel Dekker Inc., New York, 1998, p. 85.
- "The micro-scale simulation test (MST) for FCC evaluation," Akzo Nobel Symposium, Scheveningen, Netherlands, paper F-3, May 29-June 1, 1988.
- ASTM D-3907-86, "Method for testing FCC catalysts by microactivity test."
- Gerritsen, L.A., Wijngaards, H.N.J., Verwoert, J., and O'Connor, P., "Cyclic deactivation: A novel technique to simulate the deactivation of FCC catalyst in commercial units," Catal. Today, 11, 1991, p. 61.
- Pimenta R. and Imhof, P., "FCC testing philosophy," Akzo Nobel Symposium, Noordwijk, Netherlands, paper F-6, June 1998.
The authors
Ray Fletcher is the FCC technical service manager in Akzo Nobel Catalysts' Amersfoort, Netherlands, office. Previously, he worked for six years as an FCC technical service engineer in Akzo's Houston office. Mr. Fletcher has also worked for Shell Oil Co. and Texaco Refining and Marketing, Inc. He has experience in FCC, hydrotreating, catalytic reforming, alkylation, and catalytic polymerization. Mr. Fletcher has a BS in chemical engineering from the University of Washington.
Arja Hakuli is an application technologist at Akzo Nobel Catalysts in Amsterdam. She works on research and development of FCC catalysts, catalyst testing, and applications. Dr. Hakuli was previously employed by Neste Ltd., Finland, where she carried out a variety of near-refinery projects in heterogeneous catalysis. She has a PhD in chemical engineering from Helsinki University of Technology.
Pieter Imhof is head of the "Formulation and Application R&D" for FCC catalysts, Akzo Nobel Catalysts, Amsterdam. In this function, he is responsible for new catalytic materials, catalyst evaluation and test methodologies, and R&D and customer related catalyst test programs. Dr. Imhof has held various positions in FCC research and development in 10 years with Akzo Nobel. He has a PhD in organometallic and coordination chemistry from the University of Amsterdam.