New technique defines the limits of upgrading heavy oils, bitumens
A simple, new technique relates product gravity to its elemental composition in a variety of processes, using data for Alberta heavy oils and bitumens. This approach allows integration of material balances with a prediction of product quality for existing process technologies and proposed schemes.
Delayed coking and hydroconversion examples illustrate the contributions of different reactions to product characteristics and indicate the improvements in these reactions that are required to achieve significant gains in yield or product gravity.
Available and proposed processes for converting heavy oils, bitumens, and heavy residue fractions involve many simultaneous reactions, from cracking to sulfur removal. Each reaction can influence product yield and gravity, making it difficult to predict the potential of different process schemes to achieve target product quality.
Background
As heavy crudes and bitumens gain importance due to security of supply and falling reserves of light crudes, interest in field upgrading to produce higher quality products has increased.
Unlike light crudes, heavy oils and bitumens can be difficult to transport by pipeline unless they are diluted with a solvent or upgraded to reduce viscosity and increase gravity.
These transportation challenges, particularly in Canada and Venezuela, have generated tremendous interest in new upgrading processes to complement the existing coking and hydroconversion processes. Examples include obtaining hydrogen from water1 and rapid thermal cracking to maximize liquid products.2 3
Pilot plants for heavy oil processing are difficult to operate at steady state; therefore, claims of new process technologies can be difficult to assess. Any upgrading technology involves complex feed and product mixtures and multiple reactions that include cracking, hydrogenation, dehydrogenation, coke formation, and removal of sulfur and nitrogen.
Even with complete assay data, it is difficult to predict the potential of a process to give sufficient volume yield of high-gravity products. A simple correlation for Alberta crude oils allows the translation of elemental composition into density estimates. The correlation can then analyze the contribution of different reactions to improve product quality.
Given a database of density and composition data, the method can be extended to a wide range of heavy crudes and bitumens.
What limits upgrading?
The basic constraints of physics and chemistry govern the processes that convert heavy oils and bitumens into more valuable products. The first law of thermodynamics requires that total energy into a process must equal total energy out. The second law dictates that useful energy content of the products will be less than the total energy input.
Conservation of mass requires that the total mass of all products and byproducts must equal the total mass of input materials. In practical terms, difficulties in operating pilot-scale equipment make it hard to achieve closure in these balances.
While the mass of products from an upgrading process is always interesting, the major preoccupation in upgrading a heavy oil is normally the revenue received from selling the upgraded product. The market dictates that the value of a product generally increases with API gravity and with lower sulfur content.
Product density, volumetric yield, and the oil´s elemental composition are the primary determinants of potential revenue for assessment of potential new upgrading processes. Density tends to decrease with higher hydrogen content. The value of upgraded products, therefore, tends to increase with hydrogenation.
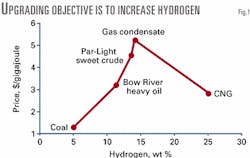
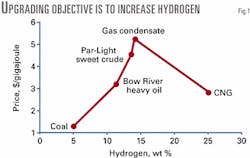
Fig. 1 illustrates this trend for Western Canada energy commodities in July 2001. The benchmark sweet crude is "Par," which normally trades close to West Texas Intermediate. The premium product in terms of price and energy content is natural gas condensate.
The next product with higher hydrogen is compressed natural gas (CNG), which is less valuable due to its limited portability. Prices for propane and butane would be between condensate and CNG.
On the low-hydrogen side, Bow River heavy oil trades at lower prices than Par, and coal is lower still. Pushing heavy oils up the price curve without going too far (beyond gas condensate) is the objective of upgrading.
The components in crude oil are predominantly non-polar hydrocarbons. Assuming that a crude oil is an ideal solution of its components is, therefore, a reasonable approximation. Excess molar enthalpy of mixing is negligible for an ideal solution; so volumes are additive.
In principle, the molar volumes of constituent components in an oil can determine its density. But due to the mixture´s complexity, this approach is not feasible. The volume occupied by an oil is, however, deterministic. The elements in an oil occupy space depending on their specific chemical bonding environment.
Tracking the possible contributions of different chemical groups to the total volume is one approach for estimating density, but it suffers from the drawback of requiring significant amounts of analytical information.4 One approach is to consider the density of simple hydrocarbons, then generalize the relationships.
Density of pure hydrocarbons
The simplest case when estimating density is that of pure hydrocarbons, which is a strong function of hydrogen content. Aromatic rings are denser and more hydrogen deficient than n-alkanes.
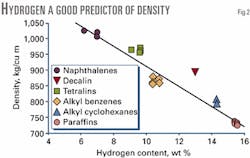
Fig. 2 shows the densities of pure hydrocarbons containing 10 and 11 carbons. Although several different structures are possible for these compounds, the density follows a reasonably linear correlation with hydrogen content on a weight basis (r2=0.93).
None of the compounds gives a significantly lower density than the correlation, although one compound falls well above the correlation (decalin or decahydro-naphthalene). Fig. 2 suggests that an approximate correlation between density and elemental composition may be useful in analyzing upgrading products.
Density of Alberta bitumens, products
In any separation or reaction process, product properties depend on the feed material and chemical nature of the process. Products from oils in a given geologic basin should contain a similar range of compound types.
Elements other than hydrogen and carbon must be considered with actual crudes. Sulfur, nitrogen, oxygen, vanadium, and nickel are present in heavy oils and may influence the feed and product density.
Data on oxygen content are rare due to the difficulty in obtaining reasonable results. Data on vanadium and nickel are available for a few materials. But they are only present in low concentrations (<250 ppm wt); so that an average molecule of feed oil will not contain metals.
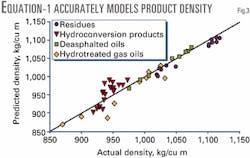
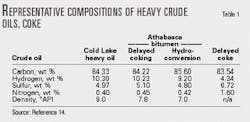
Combining data for residues from Athabasca, Cold Lake, Lloydminster and Peace River crudes, hydroconversion products, deasphalted residual oils, and hydrotreated gas oils can create correlations between composition and density.5-10 Extending the simple correlation approach to actual feeds and products results in a correlation between density and elemental analysis (Equation 1).
All three elements make a statistically significant contribution to density, and the predictions are in good agreement with actual densities (Fig. 3, multiple r2=0.95). The benefit of Equation 1 is that it considers product density as the elemental composition changes during upgrading processes.
Once density is known, API gravity can be calculated with Equation 2.
Equation 1 shows the benefit of any reaction path in terms of its effect on elemental composition. For example, release of H2S by thermal cracking will reduce the oil´s sulfur and hydrogen content.
As much as 40% of the sulfur can potentially be released as H2S in a purely thermal cracking unit, when processing 1 cu m of Cold Lake heavy oil with properties given in Table 1.11 The release of H2S reduces the sulfur content to 3.2% and gives a net hydrogen increase to 10.4% and a mass yield of 987 kg.
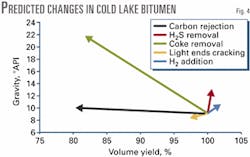
From Equation 1, the resulting density is 982 kg/cu m, or 12.6 °API. A line from the initial volume of 100 vol % and 9 °API gives the trajectory for H2S removal in the absence of any other reactions (Fig. 4).
Fig. 4 shows similar trajectories for carbon rejection (as graphite), coke rejection by delayed coking, light ends formation by thermal cracking, and hydrogen addition at a rate of 500 scf/bbl (7.5 kg/cu m). Fig. 4 is useful for considering the contributions of different reactions to the upgrading process.
Clearly, carbon rejection alone is almost completely ineffective for improving an oil´s quality because the concentration of sulfur and nitrogen in the remaining oil is more significant than the increase in hydrogen.
Fig. 4 illustrates an important point raised by Wiehe.12 Although coking is commonly described as a carbon-rejection process, the major benefit is removing such impurities as sulfur and nitrogen, as well as metals and suspended solids.
Fig. 4 also indicates that hydrogenation and the liberation of H2S are beneficial, while release of light ends is detrimental to product quality due to the loss of hydrogen in methane, ethane, etc. These trajectories are an idealized method of examining upgrading, but they do show the benefits of a particular reaction and of increasing the extent of a reaction.
For example, if sulfur removal could be extended beyond 40%, then the trajectory for this reaction would be longer, and product gravity and volumetric yield would increase.
Limits to coking performance
Any actual upgrading process will involve several simultaneous reactions. The approach in Fig. 4 can then be used to define the contribution of each reaction to product quality and to investigate potential benefits of enhancing one or more reactions.
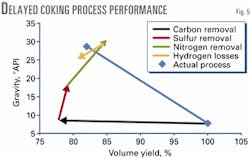
Composition changes during delayed coking provide an example. With Athabasca feed and performance listed in Table 2, the method can predict cumulative contributions of each reaction to the overall process. Fig. 5 compares the results to actual performance.
Predicted contributions of the reactions are shown as line segments, with the initial condition of 100 vol % and 7.8 °API.
Fig. 5 shows the contribution of each element, beginning with carbon removed in coke and light ends, then sulfur in coke and H2S, then nitrogen in coke, and finally hydrogen losses to coke and light ends.
The predicted product yield and density from this path is comparable to the yield of 82 vol % of 28.7 °API.
The contributions of various reactions show that sulfur and nitrogen removed in the coke have the biggest impact on product density. Higher sulfur and nitrogen removal and lower hydrogen losses increase volumetric yield and product gravity.
Reducing coke make, with the same coke composition, simply shortens the trajectories and gives a higher yield of a lower-gravity product. Removing carbon gives a small increase in density and a significant reduction in volumetric yield.
Fig. 5 does not, however, show the benefit of removing undesirable large polycyclic aromatic hydrocarbons, except where these structures result in concomitant removal of sulfur and nitrogen.
Clearly, any change in the coking process that increases sulfur and nitrogen concentration in the coke will enhance volumetric yield and product quality.
Fig. 5 indicates the magnitude of changes in various reactions to improve yield and quality. For example, if hydrogen lost to light ends and to coke were halved, the volumetric yield of liquids would increase 1.8% and product gravity would increase 2 °API.
Product oil gravity depends on its bulk elemental composition. Significant changes in upgrading reactions, therefore, are required to make a significant impact on bulk composition.
The error between predicted and actual product yield and gravity is due to two factors:
- The error inherent in the correlation of density with elemental composition (Fig. 3).
- The formation of naphtha. Materials used in correlating the density were bitumen residue fractions and gas oils but not individual naphtha fractions. The wide-boiling products from hydroconversion do not correlate as well as other samples, possibly due to the role of naphtha as a solvent.
Limits to hydrogen addition
Hydroconversion processes, such as LC-Fining and H-Oil, use catalyst and hydrogen to control coke formation and maximize liquid yields. The data of Bishop were used to calculate the individual contributions of multiple reactions to the yield and quality of the liquid products.13
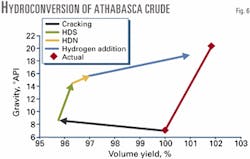
In this case, a 7 °API Athabasca bitumen with 4.75% sulfur and 0.42% nitrogen was processed via LC-Fining at the Syncrude Plant in Fort McMurray, Alta. Equation 1 gave an estimate of hydrogen content. Volume changes were relatively small, so product naphtha was an important solvent that affected the density estimates.
In the first cracking step of the predicted reaction cycle, naphtha was assumed to form at its final composition, along with the C1-C4 light ends (Fig. 6).
The remaining reactions were then assumed to occur in the gas oil and residual fractions, including hydrodesulfurization (HDS) to form H2S, and hydrodenitrogenation (HDN) to form ammonia. Adding hydrogen then served to increase the liquid products´ hydrogen content.
The separation of individual reactions that would actually occur simultaneously allows Fig. 6 to show an arbitrary reaction pathway. This approach emphasizes that detrimental material losses to light ends are made up at the expense of hydrogen addition.
Any process that minimizes the formation of light ends gains a larger net benefit from catalytically hydrogenating the oil. As with coking, removing nitrogen gives significant improvement in liquid gravity, but hydroconversion is much less effective in this regard than delayed coking.
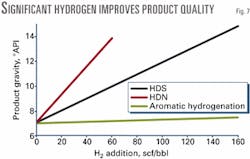
More effective catalysts for nitrogen removal would give a significant benefit in product yield. This conclusion is reinforced by Fig. 7, which shows the improvement in product gravity by adding hydrogen for HDS, HDN, and aromatics hydrogenation.
Converting sulfur to H2S increases gravity by about 5 °API/100 scf H2/bbl. Removing nitrogen is more effective because it improves gravity by 11 °API/100 scf H2/bbl, although this level of nitrogen removal is difficult to achieve with residual oils.
Adding hydrogen is less effective than heteroatom removal. This type of analysis is relevant to hydroconversion and to a variety of proposed processes to extract hydrogen from water.1 Fig. 7 suggests that while low levels of hydrogen addition may help to suppress coke formation, significant amounts of hydrogen are required to give a significant improvement in gravity even if directed only to desulfurization.
Acknowledgment
The author acknowledges Syncrude Canada Ltd. for supporting ongoing research on upgrading of bitumen.
References
- Marzin, R., Pereira, P., McGrath, M.J., Feintuch, H.M., Thompson, G., and Houde, E., "New residue process increases conversion, produces stable residue in Curacao refinery," OGJ, Nov. 2, 1998, p. 79.
- Hammond, D.G., Jacobson, M., Pagel, J.F., Poole, M.C., Green, R.C., and Serrand, W., "Fluidized bed coking process," US Patent 5658455, Aug. 19, 1997.
- Freel, B.A., and Graham, R.G., "Rapid thermal processing of heavy hydrocarbon feedstocks," Australia patent 37983A5, Nov. 14, 2000.
- Satou, M., Nemoto, H., Yokoyama, S., and Sanada, Y., "Calculation of molar volume of hydrocarbons in coal-derived liquids by a group contribution method," Energy Fuels, Vol. 5 (1991), pp. 638-42.
- Gray, M.R., Jokuty, P., Yeniova, H., Nazarewycz, L., Wanke, S.E., Achia, U., Krzywicki, A., Sanford, E.C., and Sy, O.K.Y., "The relationship between chemical structure and reactivity of Alberta bitumens," Can J. Chem. Eng., Vol. 69 (1991), pp. 833-43.
- Gray, M.R., Khorasheh, F., Wanke, S.E., Achia, U., Krzywicki, A., Sanford, E.C., Sy, O.K.Y., and Ternan, M., "The role of catalyst in hydrocracking residues from Alberta bitumens," Energy Fuels, Vol. 6 (1992), pp. 478-85.
- Oballa, M., Wong, C., Krzywicki, A., Chase, S., Dennis, G., Vandenhengel, W., and Jeffries, R., "Hydroprocessing catalyst research to support the operation of the Bi-Provincial Upgrader in Lloydminster," Proc. Int. Symp. on Heavy Oil and Residue Upgrading and Utilization, International Academic, Beijing, 1992, pp. 67-72.
- Chung, K.H., Xu, C.M., Hu, X.Y., and Wang, R.N., "Supercritical fluid extraction reveals resid properties," OGJ, Jan. 20, 1997, p. 66.
- Yui, S.M., "Hydrotreating of bitumen-derived coker gas oil: Kinetics of hydrodesulfurization, hydrodenitrogenation, and mild hydrocracking, and correlations to predict product yields and properties," AOSTRA J. Res., Vol. 5 (1989), pp. 211-24.
- Yui, S.M., "The role of catalysts in Syncrude´s operation," Can. Chem. News, July/August 1999, pp. 25-27.
- Gray, M. R., Ayasse, A.R., Chan, E.W., and Veljkovic, M., "Kinetics of desulfurization of thiophenic and sulfide sulfur in Athabasca bitumen," Energy Fuels, Vol. 9 (1995), pp. 500-06.
- Wiehe, I.A., "A phase separation kinetic model for coke formation," Ind. Eng. Chem. Res., Vol. 44 (1993), pp. 2447-57.
- Bishop, W., "LC-Finer operating experience at Syncrude," Proc. Symp. Heavy Oil: Upgrading to Refining, Can. Soc. Chem. Eng., Calgary, 1990, pp. 14-27.
- Gray, M.R., "Upgrading Petroleum Residues and Heavy Oils," New York: Marcel Dekker, 1994.
The author
Murray R. Gray holds the NSERC/Syncrude Industrial Research Chair in Advanced Upgrading of Bitumen at University of Alberta, Edmonton. His research and teaching interests focus on the processing and upgrading of heavy oils, bitumen, and petroleum residues. Gray holds a BASc in chemical engineering from University of Toronto, an MEng from University of Calgary, and a PhD in chemical engineering from California Institute of Technology, Pasadena.