Study reveals sulfidic corrosion mechanism in fired heater tubes
Hamid Rajaei
Seyed Abdolmajid Khaksar
Hassan Ghasemi
Feridun Esmaeilzadeh
Shiraz University
Shiraz, Iran
One of the most vexing problems oil and gas operations face is the failure of equipment due to corrosion. One of the most common types is sulfidic corrosion in the presence of hydrocarbon condensate.
This article describes the experience of one Iranian petrochemical company with sulfidic corrosion, which has caused the failure of fired heater tubes and led to several plant shutdowns.
To study this problem, we simulated a unit with Aspen-Hysys software to learn the effect of temperature on the corrosion rate of fired heater tubes and related piping. The simulation results are consistent with experimental ones and show that decreasing of the heater's operating temperature by 25º C., without degrading product quality, will slow the corrosion rate by 12.7 mils/year (mpy).
Common contaminant
The number of sour oil and gas fields worldwide is increasing, as sweet fields are depleted and high oil prices ensure profitable development of sour oil and gas finds.1 Sulfur is one of the most common corrosive contaminants found in high-temperature industrial environments.
Sulfur compounds originate from crude oils and include polysulfides, hydrogen sulfide, mercaptans, aliphatic sulfides, disulfides, and so forth.2 Corrosion by various sulfur compounds at temperatures between 260° and 540° C. is common in many petroleum refining processes and occasionally in petrochemical processes.3 4
During processing, the sulfur compounds decompose thermally into such constituents as hydrogen sulfide and mercaptans (organic sulfur compounds). In sufficient concentrations (greater than 0.2 wt %), these compounds are corrosive to carbon and low-alloy steel at temperatures greater than ~230-290º C. and up to 455º C.5 6 Corrosion of piping and equipment in refineries continues to be a major cause of leaks leading to equipment replacements, unplanned outages, and incidents associated with large property losses and injuries.7
The corrosion rate increases with temperature from about 450° F. (230° C.) to about 800° F. (425° C.), and at higher temperature the rate decreases.8 Also the sulfidic corrosion rate decreases in proportion to the amount of chromium in the steel.3 4 The total sulfur content includes species such as thiophenes, mercaptans, and sulfides, which show very different corrosiveness.6 It has been suggested that the mercaptans could cause higher corrosion rates than high-temperature sulfidation (by H2S) in general.9
This research focuses on simulation of a prefractionation unit in Borzouyeh Petrochemical Co.'s fourth aromatic plant in the Special Energy Zone, Assalouyeh, Iran, for mitigating sulfidic corrosion of the carbon steel heater tubes by decreasing the unit's operating temperature.
Process
The purpose of the first process unit of this plant, prefractionation, is to obtain an adequate feed for the aromizing unit by splitting the overall feed (South Pars condensate) into three fractions:
• Light ends (C6–).
• Naphtha heart cut sent to hydrotreating unit (C6–~C9).
• Heavy ends (C10+).
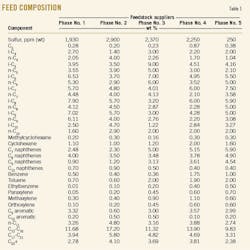
The feed stream is supplied by five South Pars natural gas processing plants. Table 1 shows the composition of the streams from the five plants named for successive phases: Phase No. 1, Phase No. 2, etc.
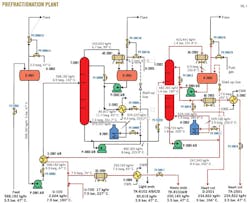
Since the feed of the plant is often supplied by gas plant Phase No. 2, this composition is selected as basis of simulations. This unit includes two splitter columns, T-2001 and T-2002 (Fig. 1).
| These two photos show the high volume of corrosion products removed from the strainer (Fig. 2a, top; Fig. 2b, bottom). |
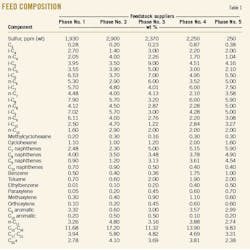
The gas condensate feed is routed to the first splitter column (T-2001) and reboiled in the first splitter reboiler (H-2001). The liquid, recovered in the first splitter reflux drum (D-2002) and called light-ends product, is cooled and sent to light-ends storage tanks. Table 2 shows the composition of this stream.
T-2001 bottoms are withdrawn and routed to the second splitter column (T-2002). This column's bottoms are reboiled in the second splitter reboiler (H-2002). The product withdrawn from the bottom of this column is referred to as heavy ends. The overhead vapors are fully condensed in the second splitter condenser (T-2002).
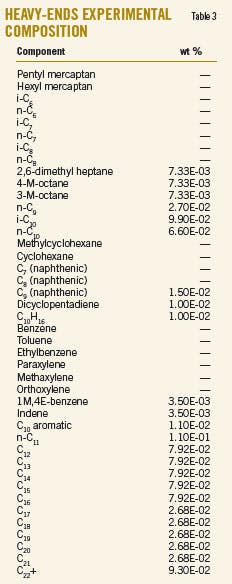
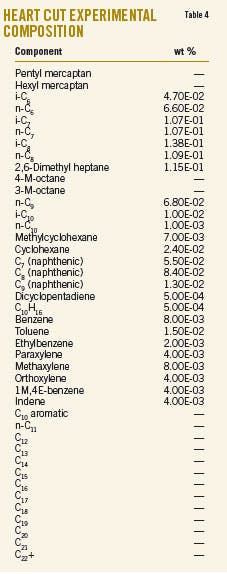
Liquid is recovered in the second splitter reflux drum (D-2003). One part of the liquid, named heart cut, is injected as reflux in T-2002, and the other part constitutes the aromizing unit feed. Tables 3 and 4 show the chemical composition of the heavy ends and the heart cut.
A big problem in this unit was the frequent plugging of the second reboiler's pump strainers due to heavy accumulation of corrosion scale, causing the unit to shut down. This problem was costly and time-consuming. Following heater (H-2002) tube failure that occurred some times after repeated plugging of the strainers, we launched an investigation to find the cause of the tube's rupture. Aspen Hysys software was also used to simulate the unit to study the possibility of reducing unit temperature without degrading product quality.
Investigation
Figs. 2 and 3 show the removed scales from the strainers and internal surfaces of the failed tubes. Fig. 3 shows severe corrosion, metal loss, and subsequent failure.
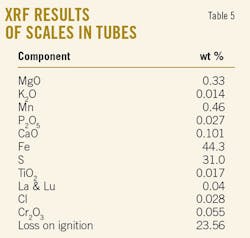
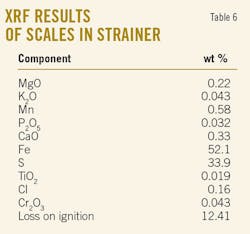
X-ray diffraction results revealed that the only main crystalline phase is FeS. Tables 5 and 6 show the results of X-ray fluorescence analyses.
Cutting the failed tubes revealed the corrosion on all internal surfaces of the tubes, and corrosion products, such as heavy scales, could be seen on the surfaces. Fig. 4 shows that the corrosion was more severe in the outlet of the tube from the heater rather than inlet.
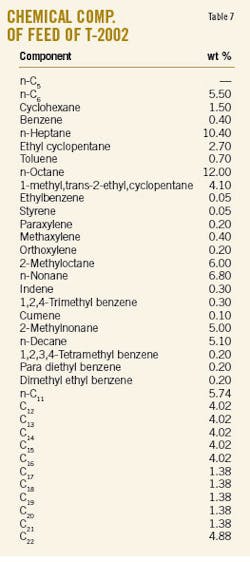
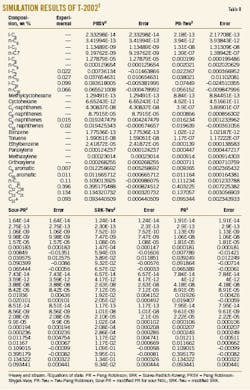
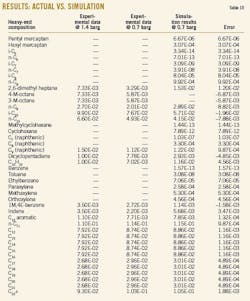
The simulation of T-2002 with Aspen-Hysys software employed several equations of states in a thermodynamic package. Table 7 shows the experimental composition of feed of T-2002. As mentioned earlier, this column has a bottom product, heavy ends, that was selected as the basis for simulation. The results of each simulation were compared to experimental data (Table 8).
The value for flash point, the most important characteristic of heavy end, must be greater than 58° C. C9 components have the most effect on the flash point of this product. Therefore, C9 components were selected as key components in this simulation. As a result, the Twu-Peng Robinson equation of state (PR-Twu) produced results the closest to the experimental data.
Change of operating conditions
As mentioned, the sulfidic corrosion rate decreases with decreasing temperature.8 Reducing the column temperature requires lowering the operating pressure of the column. We again simulated the column with Aspen-Hysys software and the PR-Twu equation of state, for all five feedstocks.
Table 9 shows the effect of pressure decrease from 1.4 barg to 0.7 barg on the bottom temperature, in two different reflux ratios. In all simulations, flash point was greater than 58° C.
The simulation results show that the 0.7-bar pressure drop will lead to about a 15° C. drop in the bottom temperature with no deviation in product quality. Moreover, due to the same economic values for both bottom and top products, the observed loss of top product flow rate is negligible. Therefore, the past operating conditions were changed to the new ones (P = 0.7 barg, bottom temperature = 228° C.). As a result, the skin temperature of the heater tubes was reduced to 313° C. from 340° C.
Table 11 compares the actual heavy-end product composition in 0.7 barg with the simulation results.
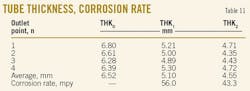
We investigated the effects on the corrosion rate of changing heater operating conditions with the tube wall thickness measurement technique. Table 11 presents the results of this test. Accordingly, the corrosion rate of the tubes in Case No. 1 (before temperature decrease) and Case No. 2 (after temperature decrease) has been calculated. Corrosion rates in the two cases were 56 mpy and 43.3 mpy, respectively. Thickness measurements at Case No. 1 (THK1) and at Case No. 2 (THK2) have been performed 12 and 18 months, respectively, after initial thickness measurement (THK0).
Decreasing of unit temperature also results in decreasing amount of the scale in the strainers.
Results
This research simulated the distillation column with Aspen-Hysys software. Comparison of simulated with experimental results showed that the PR-Twu was the best thermodynamic package for this case.
Since sulfidic corrosion depends on temperature, the operating pressure and temperature were decreased, according to the simulation results. This reduction in pressure and temperature had no negative impact on product quality.
As a result, the bottom temperature of this column and skin temperature of the fired heater tubes were reduced by 15° C. and 27° C., respectively. These changes led to a decrease of corrosion rate.
Calculated corrosion rate with tube-wall thickness measurement for Case No. 1 is 56 mpy (1.42 mm/year), which is very high, while in Case No. 2 the corrosion rate decreases to 43.3 mpy (1.1 mm/year), also decreasing the amount of corrosion products as scale in the strainer was quite obvious. A reduced corrosion rate and fewer corrosion products in the strainer indicate that decreasing of heater temperature (Case No. 2) results in reduction of the corrosion rate in the heater tubes. Although the corrosion rate in Case No. 2 is 22% less than Case No. 1, 43.3 mpy is still high.
Since changing of the feed composition or decreasing its sulfur content is not possible in the unit, as a long term solution, replacing the tubes with ones that are more corrosion resistant is the ultimate solution for avoiding corrosion problems. The sulfidic corrosion rate decreases in proportion to the amount of chromium in the steel.
Because the cessation of production due to tube failure will impose large financial losses on the company, it was preferred to replace the tubes with stainless steel ones (A 312 grade TP 321), which have good sulfidic corrosion resistance. Unit operation in Case No. 2 will be useful for decreasing corrosion rate of the stainless steel heater tubes and related carbon steel piping system.
References
1. Kvarekval, J., "Morphology of localized corrosion attacks in sour environments," NACE CORROSION/96, Denver, Mar. 24-29, 1996.
2. Lai, G.Y., editor, High-Temperature Corrosion and Materials Applications, Materials Park, Ohio: ASM International, 2007, pp. 201-10.
3. Garverick, L., editor. Corrosion in the Petrochemical Industry. Materials Park, Ohio: ASM International, 1994, pp. 330-32.
4. Davis, J.R., Heat resistant materials, Materials Park, Ohio: ASM International, 1999.
5. Tebbal, S., and Kane, R.D., "Review of Critical Factors Affecting Crude Corrosivity," NACE CORROSION/96, Denver, Mar. 24-29, 1996.
6. Shalaby, H.M., "Materials challenges in refining of heavy crude oil," KOROZYON, Vol. 15 (2007), Nos. 1-2, pp. 34-42.
7. Guidelines for avoiding sulfidation (sulfidic) corrosion failures in oil refineries, API subcommittee on corrosion & materials, RP 939-C2008.
8. Gutzeit, J., "High temperature sulfidic corrosion of steels," Process Industry Corrosion: The Theory and Practice. Houston: National Association of Corrosion Engineers, 1986.
9. An-Peter de Jong, et al., "Effect of mercaptans and other organic sulfur species on high temperature corrosion in crude and condensate distillation units," CORROSION 2007, Nashville, Mar. 11-15, 2007.
The authors
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com