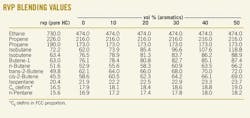
RVP blending values listed for light HCs
William Ed Smith IV
Consultant
Newark, Del.
William E. Morris
Consultant
Wilmington, Del.
Reid vapor pressure (rvp) blending values of non-aromatic hydrocarbons increase with the aromatic content of the gasoline, an aromatic effect. This effect differs for different light hydrocarbons, as was discussed in an earlier article (OGJ, Mar. 3, 2008, p. 56). That article included the effects of gasoline aromatic content on activity coefficients of individual light hydrocarbons.
Those effects are included in a chemical engineering model of the rvp test that is used to obtain rvp interaction blending equations (www.GasolineBlendingPlus.com). The earlier article did not include a list of blending values at different aromatic levels. The accompanying table here shows that list to make this technology directly useful for those planning or preparing gasoline blends.
We believe this technology will also be useful to those calculating the economic value of any combination of light hydrocarbons in gasoline.
The rvp test—ASTM D323—is no longer recognized by ASTM gasoline specification D4814 because D323 cannot be used to measure rvp of gasoline containing ethanol. Ethanol blends, however, are not shipped by all pipelines, and ethanol is limited to a maximum of 3% in some pipelines due to the risk of corrosion. The rvp test is suitable for gasoline without ethanol to be shipped by pipeline.
Gasoline rvp is controlled by the C4 and C5 hydrocarbons. As for higher boiling hydrocarbons, the rvp of a typical C6+ fraction is 2.2 lb.
With two exceptions, rvp blending values of higher boiling hydrocarbons are close to their rvp measurements. The exceptions are benzene (3.2 rvp) and toluene (1.0 rvp), which have blending values in typical gasoline of 5.4 and 1.5 lb, respectively.
The authors
William Ed Smith IV ([email protected]) is an independent consultant working in Newark, Del. He served as an officer at the Naval Radiological Defense Laboratory 1959-62 and worked for DuPont Co. 1962-93, when he retired to begin his consulting practice. Smith holds a bachelors of nuclear engineering and an MS in physics from North Carolina State University. He is a member of Sigma Xi, the Scientific Research Society.
William E. Morris ([email protected]) is a consultant in Wilmington, Del., who provides gasoline blending computer programs via www.gasolineblendingplus.com. He worked for ExxonMobil Corp.'s Bayway, NJ, refinery for 7 years. Then he joined the DuPont Co.'s petroleum laboratory where he provided technical service for refineries in support of petroleum chemical sales. Morris holds a BS in chemical engineering from the University of Missouri.